The Developmental Genetics of the Cerebellum and the Genetic Bases of Known Cerebellar Disorders
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Ultrastructural Study of the Granule Cell Domain of the Cochlear Nucleus in Rats: Mossy Fiber Endings and Their Targets
THE JOURNAL OF COMPARATIVE NEUROLOGY 369~345-360 ( 1996) Ultrastructural Study of the Granule Cell Domain of the Cochlear Nucleus in Rats: Mossy Fiber Endings and Their Targets DIANA L. WEEDMAN, TAN PONGSTAPORN, AND DAVID K. RYUGO Center for Hearing Sciences, Departments of Otolaryngoloby-Head and Neck Surgery and Neuroscience, Johns Hopkins University School of Medicine, Baltimore, Maryland 2 1205 ABSTRACT The principal projection neurons of the cochlear nucleus receive the bulk of their input from the auditory nerve. These projection neurons reside in the core of the nucleus and are surrounded by an external shell, which is called the granule cell domain. Interneurons of the cochlear granule cell domain are the target for nonprimary auditory inputs, including projections from the superior olivary complex, inferior colliculus, and auditory cortex. The granule cell domain also receives projections from the cuneate and trigeminal nuclei, which are first-order nuclei of the somatosensory system. The cellular targets of the nonprimary projections are mostly unknown due to a lack of information regarding postsynaptic profiles in the granule cell areas. In the present paper, we examined the synaptic relationships between a heterogeneous class of large synaptic terminals called mossy fibers and their targets within subdivisions of the granule cell domain known as the lamina and superficial layer. By using light and electron microscopic methods in these subdivisions, we provide evidence for three different neuron classes that receive input from the mossy fibers: granule cells, unipolar brush cells, and a previously undescribed class called chestnut cells. The distinct synaptic relations between mossy fibers and members of each neuron class further imply fundamentally separate roles for processing acoustic signals. -

Consensus Paper: Cerebellar Development
Cerebellum DOI 10.1007/s12311-015-0724-2 CONSENSUS PAPER Consensus Paper: Cerebellar Development Ketty Leto1,2 & Marife Arancillo3 & Esther B. E. Becker4 & Annalisa Buffo1,2 & Chin Chiang5 & Baojin Ding6 & William B. Dobyns 7,8 & Isabelle Dusart9,10 & Parthiv Haldipur7 & Mary E. Hatten11 & Mikio Hoshino12 & Alexandra L. Joyner13 & Masanobu Kano14 & Daniel L. Kilpatrick6 & Noriyuki Koibuchi15 & Silvia Marino16 & Salvador Martinez17 & Kathleen J. Millen7 & Thomas O. Millner16 & Takaki Miyata18 & Elena Parmigiani1,2 & Karl Schilling19 & Gabriella Sekerková20 & Roy V. Sillitoe3 & Constantino Sotelo21 & Naofumi Uesaka14 & Annika Wefers 22 & Richard J. T. Wingate23 & Richard Hawkes24 # The Author(s) 2015. This article is published with open access at Springerlink.com Abstract The development of the mammalian cerebellum is processes of cerebellar ontogenesis, highlighting the neuro- orchestrated by both cell-autonomous programs and inductive genic strategies used by developing progenitors, the genetic environmental influences. Here, we describe the main programs involved in cell fate specification, the progressive * Ketty Leto 10 Centre National de la Recherche Scientifique, CNRS, UMR8246, [email protected] INSERM U1130, Neuroscience Paris Seine, France, 75005 Paris, France 11 Laboratory of Developmental Neurobiology, The Rockefeller 1 Department of Neuroscience Rita Levi Montalcini, University of University, New York, NY 10065, USA Turin, via Cherasco 15, 10026 Turin, Italy 12 Department of Biochemistry and Cellular Biology, National Institute -

Purkinje Cell Migration Disorder By
CEREBELLAR CORTICOGENESIS IN THE LYSOSOMAL ACID PHOSPHATASE (ACP2) MUTANT MICE: PURKINJE CELL MIGRATION DISORDER BY NILOUFAR ASHTARI A Thesis Submitted to the Faculty of Graduate Studies of The University of Manitoba in Partial Fulfilment of the Requirements for the Degree of MASTER OF SCIENCE Department of Human Anatomy and Cell Science University of Manitoba Winnipeg, Manitoba Copyright © 2017 by Niloufar Ashtari 1 Abstract In a mutant mouse called nax as the result of mutation in Lysosomal Acid phosphatase (Acp2), layers of the cerebellar cortex are impaired and monolayer Purkinje cells (Pcs) turn to multi-layered Pcs that ectopically invade the molecular layer. We investigated reelin-Dab1 signaling as an important pathway for Pcs migration and monolayer formation in cerebellum. ERK1/2 is a member of mitogen activated kinases family and suggested to be a downstream of reelin signaling. We hypothesize that the establishment of mono-layered Pcs rely on reelin through ERK1/2 pathway. Acp2 mutant mice were used for this study and molecular expression and distribution were assessed by immunohistochemistry, RT-PCR, western blotting, and cell culture. Results suggest that reelin may modulate the ERK1/2 expression, thus lower expression of reelin and higher phosphorylation of Dab1 leads to over expression of the ERK1/2 that causes the Pcs to over migrate and form multilayer in nax cerebellar cortex. i TABLE OF CONTENTS LISTOFABBREVIATIONS………………………………………………..…... IV LIST OF TABLES……………………………………...…………………...….. Vii LIST OF FIGURES…………………………………………………….………. Viii CHAPTER 1: INTRODUCTION…………………………………….………… 1 1.1 Cerebellum ……………………………………………………........………. 1 1.2 Development of Central Nervous System………………………………….. 2 1.3 Development of the cerebellum………………………………….................. 3 1.4 Specification of cerebellar germinal zones…………………………………. -

Staining of Cerebellar Cortex Granular Layer Interneurons with Natural Dye of Madder Anneh Mohammad Gharravi
Gharravi Cerebellum & Ataxias (2016) 3:12 DOI 10.1186/s40673-016-0050-6 RESEARCH Open Access Staining of cerebellar cortex granular layer interneurons with natural dye of Madder Anneh Mohammad Gharravi Abstract Background: The objective of the present study was an investigation of root Rubia Tinctorum (Madder) as a natural dye to identification of granular layer interneurons of the rat cerebellum. Methods: Seven to ten micrometre sections were collected from the cerebellum and stained only with Madder for 2, 24 and 48 h. Other sections were stained with Madder then with hematoxyllin, cresyl violet, eosin, light green. Microscopic identification of cells was performed based on cell morphology, reaction and binding of with the dye. All data were expressed as mean ± SD in and significance was set at p ≤0.05. Results: Madder with alum as mordant resulted a deep red staining of interneurons. Unipolar brush cells (UBCs) were observed with a cell body diameter intermediate between granule and Golgi cells in the superficial layer of the granular layer. Golgi cells were identified almost as large as Purkinje cells with irregular rounded or polygonal morphology. Lugaro cells were observed as spindle-shaped cells adjacent to Purkinje layer. Conclusion: Results of the present study showed that mader could stain granular layer interneurons in cerebellum cortex of rat. Keywords: Madder, Cerebellum, Unipolar brush cells, Lugaro cell, Golgi neurons Background with all cerebellar cortical neurons and fibers and they Histologically, the cerebellar cortex is divided into three function as inhibitory interneurons. These spindle-shaped layers: the molecular, the Purkinje and the granular cells locate just underneath the Purkinje cell layer are layers. -

A Model of Long-Term Memory Storage in the Cerebellar Cortex: a Possible Role for Plasticity at Parallel Fiber Synapses Onto Stellate͞basket Interneurons
Proc. Natl. Acad. Sci. USA Vol. 94, pp. 14200–14205, December 1997 Psychology A model of long-term memory storage in the cerebellar cortex: A possible role for plasticity at parallel fiber synapses onto stellateybasket interneurons GARRETT T. KENYON† Department of Neurobiology and Anatomy, University of Texas Medical School at Houston, 6431 Fannin, Houston, TX 77030 Edited by Richard F. Thompson, University of Southern California, Los Angeles, CA, and approved October 3, 1997 (received for review April 14, 1997) ABSTRACT By evoking changes in climbing fiber activity, Despite evidence supporting their postulated role in motor movement errors are thought to modify synapses from parallel learning, theoretical arguments suggest that memories stored fibers onto Purkinje cells (pf*Pkj) so as to improve subse- at pf*Pkj synapses would be susceptible to long-term degra- quent motor performance. Theoretical arguments suggest dation as a result of ongoing motor adaptation. First, motor there is an intrinsic tradeoff, however, between motor adap- memories are likely to be distributed across overlapping sets of tation and long-term storage. Assuming a baseline rate of pf*Pkj synapses. If motor memories did not overlap, the motor errors is always present, then repeated performance of storage capacity of pf*Pkj synapses would be greatly reduced any learned movement will generate a series of climbing (12, 17). Second, it is reasonable to assume that the pattern of fiber-mediated corrections. By reshuffling the synaptic synaptic weights necessary to effect any given movement is weights responsible for any given movement, such corrections non-unique. If the activity of a Purkinje cell depends only on will degrade the memories for other learned movements stored the total sum of the synaptic input at any given moment, then in overlapping sets of synapses. -

FIRST PROOF Cerebellum
Article Number : EONS : 0736 GROSS ANATOMY Cerebellum Cortex The cerebellar cortex is an extensive three-layered sheet with a surface approximately 15 cm laterally THE HUMAN CEREBELLUM (‘‘little brain’’) is a and 180 cm rostrocaudally but densely folded around significant part of the central nervous system both three pairs of nuclei. The cortex is divided into three in size and in neural structure. It occupies approxi- transverse lobes: Anterior and posterior lobes are mately one-tenth of the cranial cavity, sitting astride separated by the primary fissure, and the smaller the brainstem, beneath the occipital cortex, and flocculonodular lobe is separated by the poster- contains more neurons than the whole of the cerebral olateral fissure (Fig. 1). The anterior and posterior cortex. It consists of an extensive cortical sheet, lobes are folded into a number of lobules and further densely folded around three pairs of nuclei. The folded into a series of folia. This transverse organiza- cortex contains only five main neural cell types and is tion is then divided at right angles into broad one of the most regular and uniform structures in the longitudinal regions. The central vermis, named for central nervous system (CNS), with an orthogonal its worm-like appearance, is most obvious in the ‘‘crystalline’’ organization. Major connections are posterior lobe. On either side is the paravermal or made to and from the spinal cord, brainstem, and intermediate cortex, which merges into the lateral sensorimotor areas of the cerebral cortex. hemispheres. The most common causes of damage to the cerebellum are stroke, tumors, or multiple sclerosis. -

The Stellate Cells of the Rat's Cerebellar Cortex*
Z. Anat. Entwickl.-Gesch. 136, 224--248 (1972) by Springer-Verlag 1972 The Stellate Cells of the Rat's Cerebellar Cortex* Victoria Chan-Palay and Sanford L. Palay Departments of Neurobiology and Anatomy, Harvard Medical School, Boston, Massachusetts Received February 18, 1972 Summary. Stellate cells were studied in rapid Golgi preparations and in electron micro- graphs. These small neurons can be classified on the basis of their position in the molecular layer and the patterns of their dendritic and axonal arborizations as follows: (1) superficial cells with short, contorted dendrites and a circumscribed axonal arbor (upper third of the molecular layer) ; (2) deep stellate cells with radiating, twisted dendrites and with long axons giving rise to thin, varicose collaterals (middle third of the molecular layer); (3) deep stellate cells with similar dendrites and long axons giving collaterals to the basket around the Purkinje cell bodies (middle third of the molecular layer). An important characteristic of the stellate cell axon is that it generates most of its collaterals close to its origin. Even in long axon cells, only a few collaterals issue from the more distant parts of the axon. These forms contrast with the basket cell, which sends out long, straighter dendrites, and an extended axon that first emits branches at some distance from its origin. Furthermore, basket cell axon collaterals are usually stout in contrast to the frail, beaded collaterals of the stellate cell axon. The two cell types are considered to be distinct. In electron micrographs stellate cells display folded nuclei and sparse cytoplasm with the characteristics usual for small neurons. -

Forward Signaling by Unipolar Brush Cells in the Mouse Cerebellum
Cerebellum DOI 10.1007/s12311-015-0693-5 ORIGINAL PAPER Forward Signaling by Unipolar Brush Cells in the Mouse Cerebellum Stijn van Dorp1 & Chris I. De Zeeuw 1,2 # The Author(s) 2015. This article is published with open access at Springerlink.com Abstract Unipolar brush cells (UBCs) are glutamatergic in- Introduction terneurons prominently present in the granular layer of the vestibulocerebellum. UBCs engage in extensive synaptic con- Forward processing, in particular the absence of recurrent tact with a single presynaptic mossy fiber and signal to down- excitation, is a defining feature of cerebellar architecture stream granule cells through an elaborate network of mossy and computation. In the granular layer of the fiber-like axons. Ultrastructural examinations and electro- vestibulocerebellum, unipolar brush cells (UBCs) provide physiological recordings in organotypic slice cultures have a powerful forward excitatory action onto granule cells indicated that UBCs target not only granule cells but also other through a cortex-intrinsic network of mossy fiber-like UBCs, thus forming chains of two or perhaps more intercon- axons [1, 2]. UBCs are characterized by an elaborate nected UBCs. In this report, we show recordings of spontane- brush-like dendrite (Fig. 1a) that forms an unusually exten- ous and evoked (di)synaptic events in granule cells and UBCs sive synaptic contact with a single presynaptic mossy fiber in fresh cerebellar slices from juvenile mice (5–7 weeks). The rosette [3]. This highly specialized configuration has been patterns of arrival of synaptic events were consistent with the proposed to facilitate prolonged entrapment of glutamate presence of a presynaptic UBC, and recordings from UBCs in the synaptic cleft, underlying complex temporal trans- displayed spontaneous protracted synaptic events characteris- formations of incoming mossy fiber signals [4, 5]. -
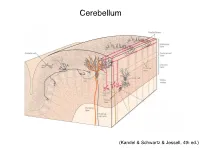
Cerebellum and Activation of the Cerebellum IO ST During Nonmotor Tasks
Cerebellum (Kandel & Schwartz & Jessell, 4th ed.) Granule186 cells are irreducibly smallChapter (6-8 7 μm) stellate cell basket cell outer synaptic layer PC rs cap PC layer grc Go inner 6 μm synaptic layer Figure 7.15 Largest cerebellar neuron occupies more than a 1,000-fold greater volume than smallest neuron. Thin section (~1 μ m) through monkey cerebellar cortex. Purkinje cell body (PC) and nucleus are far larger than those of granule cell (grc). The latter cluster to leave space for mossy fiber terminals to form glomeruli with grc dendritic claws and space for Golgi cells (Go). Note rich network of capillaries (cap). Fine, scat- tered dots are mitochondria. Courtesy of E. Mugnaini. brain ’ s most numerous neuron, this small cost grows large (see also chapter 13). Much of the inner synaptic layer is occupied by the large axon terminals of mossy fibers (figures 7.1 and 7.16). A terminal interlaces with multiple (~15) dendritic claws, each from a different but neighboring granule cell, and forms a complex knot (glomerulus ), nearly as large as a granule cell body (figures 7.1 and 7.16). The mossy fiber axon fires at an unusually high mean rate (up to 200 Hz) and is therefore among the brain’ s thickest (figure 4.6). To match the axon’ s high rate, a terminal expresses 150 active zones, 10 per postsynaptic granule cell (figure 7.16). These sites are capable of driving … and most expensive. 190 Chapter 7 10 4 8 3 9 19 10 10 × 6 × 2 2 4 ATP/s/cell ATP/s/cell ATP/s/m 1 2 0 0 astrocyte astrocyte Golgi cell Golgi cell basket cell basket cell stellate cell stellate cell granule cell granule cell mossy fiber mossy fiber Purkinje cell Purkinje Purkinje cell Purkinje Bergman glia Bergman glia climbing fiber climbing fiber Figure 7.18 Energy costs by cell type. -

Early Onset of Ataxia in Moonwalker Mice Is Accompanied by Complete Ablation of Type II Unipolar Brush Cells and Purkinje Cell Dysfunction
The Journal of Neuroscience, December 11, 2013 • 33(50):19689–19694 • 19689 Brief Communications Early Onset of Ataxia in Moonwalker Mice Is Accompanied by Complete Ablation of Type II Unipolar Brush Cells and Purkinje Cell Dysfunction Gabriella Sekerkova´,1,2* Jin-Ah Kim,1* Maximiliano J. Nigro,1 Esther B. E. Becker,3 Jana Hartmann,4 Lutz Birnbaumer,5 Enrico Mugnaini,1,2 and Marco Martina1 Departments of 1Physiology and 2Cell and Molecular Biology, Northwestern University Feinberg School of Medicine, Chicago, Illinois 60611, 3Department of Physiology, Anatomy, and Genetics, MRC Functional Genomics Unit, Oxford OX1 3PT, United Kingdom, 4Institute of Neuroscience, Technical University Munich, 80802 Munich, Germany, and 5Laboratory of Neurobiology, National Institute of Environmental Health Sciences, National Institutes of Health, Department of Health and Human Services, Durham, North Carolina 27709 Transient receptor potential “canonical” cation channels (TRPC) are involved in many cellular activities, including neuronal synaptic transmission. These channels couple lipid metabolism, calcium homeostasis, and electrophysiological properties as they are calcium permeable and activated through the phospholipase C pathway and by diacylglycerol. The TRPC3 subunit is abundantly expressed in Purkinje cells (PCs), where it mediates slow metabotropic glutamate receptor-mediated synaptic responses. Recently, it has been shown that heterozygous moonwalker mice, which are a model of cerebellar ataxia, carry a dominant gain-of-function mutation (T635A) in the TRPC3 gene. This mutation leads to PC loss and dysmorphism, which have been suggested to cause the ataxia. However, the ataxic phenotype is present from a very early stage (before weaning), whereas PC loss does not appear until several months of age. -

An Updated Investigation on the Dromedary Camel Cerebellum (Camelus Dromedarius) with Special Insight Into the Distribution of Calcium‑Binding Proteins Abdelraheim H
www.nature.com/scientificreports OPEN An updated investigation on the dromedary camel cerebellum (Camelus dromedarius) with special insight into the distribution of calcium‑binding proteins Abdelraheim H. Attaai1,3, Ahmed E. Noreldin2, Fatma M. Abdel‑maksoud1* & Manal T. Hussein1,3 Studying the cerebella of diferent animals is important to expand the knowledge about the cerebellum. Studying the camel cerebellum was neglected even though the recent research in the middle east and Asia. Therefore, the present study was designed to achieve a detailed description of the morphology and the cellular organization of the camel cerebellum. Because of the high importance of the calcium ions as a necessary moderator the current work also aimed to investigate the distribution of calcium binding proteins (CaBP) such as calbindin D‑28K (CB), parvalbumin (PV) and calretinin (CR) in diferent cerebellar cells including the non‑traditional neurons. The architecture of camel cerebellum, as diferent mammals, consists of the medulla and three layered‑cortex. According to our observation the cells in the granular layer were not crowded and many spaces were observed. CB expression was the highest by Purkinje cells including their dendritic arborization. In addition to its expression by the inhibitory interneurons (basket, stellate and Golgi neurons), it is also expressed by the excitatory granule cells. PV was expressed by Purkinje cells, including their primary arborization, and by the molecular layer cells. CR immunoreactivity (‑ir) was obvious in almost all cell layers with varying degrees, however a weak or any expression by the Purkinje cells. The molecular layer cells and the Golgi and the non traditional large neurons of the granular layer showed the strongest CR‑ir. -

Sensing How to Balance
INSIGHT CEREBELLUM Sensing how to balance How does the inner ear communicate with the cerebellar cortex to maintain balance and posture? FABRICE ANGO AND RAPHAE¨ L DOS REIS Both the primary and secondary afferents Related research article Balmer TS, Trus- form synapses with neurons called granule cells sell LO. 2019. Selective targeting of unipo- in the cerebellum: granule cells are the most lar brush cell subtypes by cerebellar mossy numerous excitatory neurons in the brain fibers. eLife 8:e44964. DOI: 10.7554/eLife. (Chadderton et al., 2004). A single mossy fiber 44964 can activate hundreds of granule cells which, in turn, form synapses with the dendrites of Pur- kinje cells. These cells are the sole output neu- rons from the cerebellar cortex and they have a crucial role in motor learning. eeping your head upright may seem like However, this is not the full story because the a trivial task, but the neural circuitry vestibular region of the cerebellum also contains K required to perform this task is rather a high proportion of excitatory neurons called complex and not fully understood. This circuitry unipolar brush cells (UBCs). These cells, which starts with the vestibular system: a sensory sys- receive input from just a single mossy fiber, form tem in the inner ear that relies on hair cells to synapses with the granule cells (Mugnaini et al., 2011). UBCs essentially create an intermediate detect movements, and to provide our sense of step in the circuitry, where signals sent between balance and spatial awareness. The vestibular mossy fibers and granule cells can be modified.