The Stellate Cells of the Rat's Cerebellar Cortex*
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

A Model of Long-Term Memory Storage in the Cerebellar Cortex: a Possible Role for Plasticity at Parallel Fiber Synapses Onto Stellate͞basket Interneurons
Proc. Natl. Acad. Sci. USA Vol. 94, pp. 14200–14205, December 1997 Psychology A model of long-term memory storage in the cerebellar cortex: A possible role for plasticity at parallel fiber synapses onto stellateybasket interneurons GARRETT T. KENYON† Department of Neurobiology and Anatomy, University of Texas Medical School at Houston, 6431 Fannin, Houston, TX 77030 Edited by Richard F. Thompson, University of Southern California, Los Angeles, CA, and approved October 3, 1997 (received for review April 14, 1997) ABSTRACT By evoking changes in climbing fiber activity, Despite evidence supporting their postulated role in motor movement errors are thought to modify synapses from parallel learning, theoretical arguments suggest that memories stored fibers onto Purkinje cells (pf*Pkj) so as to improve subse- at pf*Pkj synapses would be susceptible to long-term degra- quent motor performance. Theoretical arguments suggest dation as a result of ongoing motor adaptation. First, motor there is an intrinsic tradeoff, however, between motor adap- memories are likely to be distributed across overlapping sets of tation and long-term storage. Assuming a baseline rate of pf*Pkj synapses. If motor memories did not overlap, the motor errors is always present, then repeated performance of storage capacity of pf*Pkj synapses would be greatly reduced any learned movement will generate a series of climbing (12, 17). Second, it is reasonable to assume that the pattern of fiber-mediated corrections. By reshuffling the synaptic synaptic weights necessary to effect any given movement is weights responsible for any given movement, such corrections non-unique. If the activity of a Purkinje cell depends only on will degrade the memories for other learned movements stored the total sum of the synaptic input at any given moment, then in overlapping sets of synapses. -

FIRST PROOF Cerebellum
Article Number : EONS : 0736 GROSS ANATOMY Cerebellum Cortex The cerebellar cortex is an extensive three-layered sheet with a surface approximately 15 cm laterally THE HUMAN CEREBELLUM (‘‘little brain’’) is a and 180 cm rostrocaudally but densely folded around significant part of the central nervous system both three pairs of nuclei. The cortex is divided into three in size and in neural structure. It occupies approxi- transverse lobes: Anterior and posterior lobes are mately one-tenth of the cranial cavity, sitting astride separated by the primary fissure, and the smaller the brainstem, beneath the occipital cortex, and flocculonodular lobe is separated by the poster- contains more neurons than the whole of the cerebral olateral fissure (Fig. 1). The anterior and posterior cortex. It consists of an extensive cortical sheet, lobes are folded into a number of lobules and further densely folded around three pairs of nuclei. The folded into a series of folia. This transverse organiza- cortex contains only five main neural cell types and is tion is then divided at right angles into broad one of the most regular and uniform structures in the longitudinal regions. The central vermis, named for central nervous system (CNS), with an orthogonal its worm-like appearance, is most obvious in the ‘‘crystalline’’ organization. Major connections are posterior lobe. On either side is the paravermal or made to and from the spinal cord, brainstem, and intermediate cortex, which merges into the lateral sensorimotor areas of the cerebral cortex. hemispheres. The most common causes of damage to the cerebellum are stroke, tumors, or multiple sclerosis. -
Homo Sapiens (445) MEDIUM (=IQR) HIGH
Dataset: 445 anatomical parts from data selection: HS_mRNASeq_HUMAN_GL-1 Showing 2 measure(s) of 2 gene(s) on selection: HS-0 TMPRSS2 ACE2 Level of expression Homo sapiens (445) MEDIUM (=IQR) HIGH 0 1 2 3 4 5 6 7 8 9 10 11 samples avg. expr. Tissue 3435 1.42 gestational structure 4 1.92 extraembryonic tissue / fluid 4 1.92 placenta 4 1.92 alimentary system 721 3.93 gastrointestinal tract 519 4.83 mouth (oral cavity) 7 0.87 oral mucosa (unspecified) 4 1.13 salivary gland 3 0.54 stomach 34 2.82 gastric (stomach) region 31 2.90 stomach cardia 11 2.13 stomach body 11 4.06 pyloric antrum 9 2.42 intestine 463 5.14 large intestine 294 3.48 caecum (cecum) 7 3.17 apex of caecum (appendix) 3 1.96 colorectum 287 3.49 colon 207 3.60 colonic mucosa 84 3.43 proximal colon 35 3.20 proximal colonic mucosa 35 3.20 ascending colon 30 3.32 transverse colon 5 2.53 distal colon 31 2.94 distal colonic mucosa 31 2.94 descending colon 6 2.51 sigmoid colon 25 3.05 rectosigmoid colon 5 2.33 rectosigmoid junction 6 3.47 rectum 62 3.00 small intestine 168 8.07 ileum 157 8.36 ileal mucosa 76 8.78 esophagus (oesophagus) 15 1.60 liver and biliary system 130 1.50 liver 119 1.36 gall bladder 3 6.94 bile duct 8 1.56 intrahepatic bile duct 8 1.56 pancreas 72 1.83 pancreatic islet (islet of Langerhans) 70 1.87 circulatory system 791 0.21 cardiovascular system 26 3.51 heart 19 3.39 heart muscle (myocardium, cardiac muscle) 7 4.10 heart ventricle 3 3.88 heart left ventricle 3 3.88 left ventricle free wall 3 3.88 vascular system 7 3.83 blood vessel 7 3.83 artery 7 3.83 coronary -

The Developmental Genetics of the Cerebellum and the Genetic Bases of Known Cerebellar Disorders
THE DEVELOPMENTAL GENETICS OF THE CEREBELLUM AND THE GENETIC BASES OF KNOWN CEREBELLAR DISORDERS A Review of Developmental Genetics of the Cerebellum and the Genetic Bases of known Cerebellar Disorders Olakada Favour Adamba 17/MHS01/254 Department of Medicine and Health Sciences College of Medicine and Health Sciences Afe Babalola University ANA 303: Neuroanatomy July, 2020 The Developmental Genetics of Cerebellum and the Genetic Bases of known Cerebellar Disorders: A Literature Review Olakada Favour Adamba1 Abstract The internal structure of the cerebellum is an intriguing paradox; its cytoarchitecture is relatively simple compared to the connections between its neurons, which are wired into a complex array of gene expression domains and functional circuits. The genetic research of cerebellar development has provided a great deal of information about the molecular events directing the formation of the cerebellum. The developmental mechanisms that coordinate the establishment of cerebellar structure and circuitry provide a powerful model for understanding how a functional brain develops and its significance in cerebellar disorders and diseases. The cellular makeup of the cerebellum is derived from two primary germinal matrices (the ventricular zone and a specialized germinal matrix called the rhombic lip). Each matrix/zone expresses a specific set of genes that establish the cell lineages within the cerebellar anlage. Then, cohorts of differentiated projection neurons and interneuron progenitors migrate into the developing cerebellum. thereafter, a number of remarkable patterning events occur. Altogether, structural and molecular organisations are thought to support the proper connectivity between incoming afferent projections and their target cells. Key words: Cerebellum, circuitry, genetic, development, disorders. I. Introduction The cerebellum (‘little brain’) resides at the anterior end of the hindbrain and is classically defined by its role in sensory-motor processing (Buckner, 2013). -
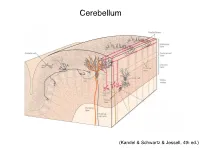
Cerebellum and Activation of the Cerebellum IO ST During Nonmotor Tasks
Cerebellum (Kandel & Schwartz & Jessell, 4th ed.) Granule186 cells are irreducibly smallChapter (6-8 7 μm) stellate cell basket cell outer synaptic layer PC rs cap PC layer grc Go inner 6 μm synaptic layer Figure 7.15 Largest cerebellar neuron occupies more than a 1,000-fold greater volume than smallest neuron. Thin section (~1 μ m) through monkey cerebellar cortex. Purkinje cell body (PC) and nucleus are far larger than those of granule cell (grc). The latter cluster to leave space for mossy fiber terminals to form glomeruli with grc dendritic claws and space for Golgi cells (Go). Note rich network of capillaries (cap). Fine, scat- tered dots are mitochondria. Courtesy of E. Mugnaini. brain ’ s most numerous neuron, this small cost grows large (see also chapter 13). Much of the inner synaptic layer is occupied by the large axon terminals of mossy fibers (figures 7.1 and 7.16). A terminal interlaces with multiple (~15) dendritic claws, each from a different but neighboring granule cell, and forms a complex knot (glomerulus ), nearly as large as a granule cell body (figures 7.1 and 7.16). The mossy fiber axon fires at an unusually high mean rate (up to 200 Hz) and is therefore among the brain’ s thickest (figure 4.6). To match the axon’ s high rate, a terminal expresses 150 active zones, 10 per postsynaptic granule cell (figure 7.16). These sites are capable of driving … and most expensive. 190 Chapter 7 10 4 8 3 9 19 10 10 × 6 × 2 2 4 ATP/s/cell ATP/s/cell ATP/s/m 1 2 0 0 astrocyte astrocyte Golgi cell Golgi cell basket cell basket cell stellate cell stellate cell granule cell granule cell mossy fiber mossy fiber Purkinje cell Purkinje Purkinje cell Purkinje Bergman glia Bergman glia climbing fiber climbing fiber Figure 7.18 Energy costs by cell type. -

The Modifiable Neuronal Network of the Cerebellum
Japanese Journal of Physiology, 34, 781-792, 1984 MINIREVIEW The Modifiable Neuronal Network of the Cerebellum M asao ITO Department of Physiology, Faculty of Medicine, University of Tokyo, Bunkyo-ku, Tokyo, 113 Japan An outstanding feature of the cerebellum known since the classic histological work of Ramon y Cajal is the relative simplicity and highly ordered geometry of its neuronal network. The cerebellar neuronal network consists of five major types of cortical neurons (Purkinje, basket, stellate, Golgi, and granule cells), two major types of afferents (mossy and climbing fibers), and the subcortical cells in the vestibular and cerebellar nuclei. When the neuronal network structure of the cerebellum was comprehensively dissected and described in great detail in the 1960s, and summarized by ECCLES,ITO, and SZ.ENTAGOTHAI[8], it was taken as a challenge to understand more completely the complex functions of the central nervous system using these fundamental studies of the brain as groundwork for future elucidation. As the gap between our understanding of the brain and our knowledge of neuronal networks is in general so large, hope has arisen that cere- bellar physiology may successfully fill the gap rather soon. Rigorous efforts using numerous newly-introduced techniques have been devoted during the past two decades, yielding remarkable progress that meets expectations at least to some extent. A number of problems remain to be solved, however, before we fully understand the cerebellum. It seems important at this point to review the major achievements of the past two decades in order to gain a clearer perspective of cerebellar physiology for the coming decade. -

An Updated Investigation on the Dromedary Camel Cerebellum (Camelus Dromedarius) with Special Insight Into the Distribution of Calcium‑Binding Proteins Abdelraheim H
www.nature.com/scientificreports OPEN An updated investigation on the dromedary camel cerebellum (Camelus dromedarius) with special insight into the distribution of calcium‑binding proteins Abdelraheim H. Attaai1,3, Ahmed E. Noreldin2, Fatma M. Abdel‑maksoud1* & Manal T. Hussein1,3 Studying the cerebella of diferent animals is important to expand the knowledge about the cerebellum. Studying the camel cerebellum was neglected even though the recent research in the middle east and Asia. Therefore, the present study was designed to achieve a detailed description of the morphology and the cellular organization of the camel cerebellum. Because of the high importance of the calcium ions as a necessary moderator the current work also aimed to investigate the distribution of calcium binding proteins (CaBP) such as calbindin D‑28K (CB), parvalbumin (PV) and calretinin (CR) in diferent cerebellar cells including the non‑traditional neurons. The architecture of camel cerebellum, as diferent mammals, consists of the medulla and three layered‑cortex. According to our observation the cells in the granular layer were not crowded and many spaces were observed. CB expression was the highest by Purkinje cells including their dendritic arborization. In addition to its expression by the inhibitory interneurons (basket, stellate and Golgi neurons), it is also expressed by the excitatory granule cells. PV was expressed by Purkinje cells, including their primary arborization, and by the molecular layer cells. CR immunoreactivity (‑ir) was obvious in almost all cell layers with varying degrees, however a weak or any expression by the Purkinje cells. The molecular layer cells and the Golgi and the non traditional large neurons of the granular layer showed the strongest CR‑ir. -

Spontaneous Activity Signatures of Morphologically Identified Interneurons in the Vestibulocerebellum
712 • The Journal of Neuroscience, January 12, 2011 • 31(2):712–724 Behavioral/Systems/Cognitive Spontaneous Activity Signatures of Morphologically Identified Interneurons in the Vestibulocerebellum Tom J. H. Ruigrok,1 Robert A. Hensbroek,2 and John I. Simpson2 1Department of Neuroscience, Erasmus Medical Center Rotterdam, 3000 CA Rotterdam, The Netherlands, and 2Department of Physiology and Neuroscience, New York University School of Medicine, New York, New York 10016 Cerebellar cortical interneurons such as Golgi cells, basket cells, stellate cells, unipolar brush cells, and granule cells play an essential role in the operations of the cerebellum. However, detailed functional studies of the activity of these cells in both anesthetized and behaving animals have been hampered by problems in recognizing their physiological signatures. We have extracellularly recorded the spontane- ous activity of vestibulocerebellar interneurons in ketamine/xylazine-anesthetized rats and subsequently labeled them with Neurobiotin using the juxtacellular technique. After recovery and morphological identification of these cells, they were related to statistical measures of their spontaneous activity. Golgi cells display a somewhat irregular firing pattern with relatively low average frequencies. Unipolar brush cells are characterized by more regular firing at higher rates. Basket and stellate cells are alike in their firing characteristics, which mainly stand out by their irregularity; some of them are set apart by their very slow average rate. The spontaneous activity of interneurons examined in the ketamine/xylazine rabbit fit within this general pattern. In the rabbit, granule cells were identified by the spontaneous occurrence of extremely high-frequency bursts of action potentials, which were also recognized in the rat. -

Stellate Cell Computational Modeling Predicts Signal Filtering in the Molecular Layer Circuit of Cerebellum
www.nature.com/scientificreports OPEN Stellate cell computational modeling predicts signal fltering in the molecular layer circuit of cerebellum Martina Francesca Rizza1, Francesca Locatelli1, Stefano Masoli1, Diana Sánchez‑Ponce3, Alberto Muñoz3,4, Francesca Prestori1,5 & Egidio D’Angelo1,2,5* The functional properties of cerebellar stellate cells and the way they regulate molecular layer activity are still unclear. We have measured stellate cells electroresponsiveness and their activation by parallel fber bursts. Stellate cells showed intrinsic pacemaking, along with characteristic responses to depolarization and hyperpolarization, and showed a marked short‑term facilitation during repetitive parallel fber transmission. Spikes were emitted after a lag and only at high frequency, making stellate cells to operate as delay‑high‑pass flters. A detailed computational model summarizing these physiological properties allowed to explore diferent functional confgurations of the parallel fber—stellate cell—Purkinje cell circuit. Simulations showed that, following parallel fber stimulation, Purkinje cells almost linearly increased their response with input frequency, but such an increase was inhibited by stellate cells, which leveled the Purkinje cell gain curve to its 4 Hz value. When reciprocal inhibitory connections between stellate cells were activated, the control of stellate cells over Purkinje cell discharge was maintained only at very high frequencies. These simulations thus predict a new role for stellate cells, which could endow the molecular layer with low‑pass and band‑pass fltering properties regulating Purkinje cell gain and, along with this, also burst delay and the burst‑pause responses pattern. Te stellate cells (SCs) are inhibitory interneurons of the cerebellum molecular layer (ML) that were frst identi- fed in histological preparations by Golgi 1 and Cajal2,3. -

C-Kit Receptor and Ligand Expression in Postnatal Development of the Mouse Cerebellum Suggests a Function for C-Kif in Inhibitory Interneurons
The Journal of Neuroscience, December 1992, 72(12): 4663-4676 c-kit Receptor and Ligand Expression in Postnatal Development of the Mouse Cerebellum Suggests a Function for c-kif in Inhibitory Interneurons Katia Manova,1~2~a Rosemary F. Bachvarova,* Eric J. Huang,’ Sandra Sanchez,’ Stephen M. Pronovost,l Eric Velazquez,113 Barbara McGuire,*v3 and Peter Besmerl ‘Molecular Biology Department, Sloan Kettering Institute and Cornell University Graduate School of Medical Sciences, New York, New York 10021, *Department of Cell Biology and Anatomy, Cornell University Medical College, New York, New York 10021, and 3Laboratory of Neurobiology, Rockefeller University, New York, New York 10021 The c-kit receptor and its cognate ligand, KL, are encoded Increasing evidence implicates several tyrosine kinase receptor at the white spoffjng locus (IW) and the steel locus (9) of systems in neurogenic processes.NGF, brain-derived neuro- the mouse, respectively. S/and Wmutations affect the same trophic factor, and neurotrophin-3 were recently shown to be cellular targets in melanogenesis, gametogenesis and he- ligandsof the c-trk, c-trkB, and c-trkC tyrosine kinase receptors, matopoiesis during embryonic development and in adult life. respectively (Kaplan et al., 1991; Lamballe et al., 199 1; Squint0 c-kit is expressed in cellular targets of Wand SI mutations, et al., 1991). These factors regulate cell survival, differentiation, whereas KL is expressed in the microenvironment of these and neurotransmitter synthesisof distinct sets of neurons (for targets. c-kitand KL, however, are also expressed in tissues a review, seeBarde, 1989). In vitro, basicfibroblast growth factor and cell types that are not targets of W and Sl mutations, enhancesthe survival and differentiation of granule neurons including the brain. -

Subcortical Motor Systems: Cerebellum
NN 23 Outline Anatomy Subcortical Motor Cerebellar cortex Neuronal circuitry Systems: cerebellum Cerebellar connections 陽明大學醫學院 腦科所 Vestibulocerebellum Spinocerebellum 陳昌明 副教授 Neocerebellum Other cerebellar functions Motor Loops Pyramidal Tract and Associated Circuits Cortex ---> Subcortex ---> Cortex ---> Spinal cord upper motor neuron UMN Cerebellum coordination of movement Cerebellum BASAL Basal Ganglia GANGLIA pyramidal tract selection & initiation of voluntary movements ~ lower motor neuron UMN Cerebellar functions Cerebellum: Anatomy The main functions: Coordinating skilled voluntary movements by influencing muscle activity, Controlling equilibrium and muscle tone through connections with the vestibular system and the spinal cord and its gamma motor neurons. 1. Found in the posterior Cranial fossa 2. Forms a roof over the 4th ventricle 3. Lies above and behind the medullar and pons 1 NN 23 Cerebellum: Anatomy Cerebellum: External features Folia & lobules Longitudinal division analogous to gyrus Vermis, Paravermal Vermis - along midline Region, Cerebellar Hemisphere output ---> ventromedial pathway Transverse division Hemispheres Anterior Lobe Posterior Lobe output ---> lateral pathway Flocculonodular Deep cerebellar nuclei Lobe fastigial, interposed, & dentate Major output structures ~ Functional divisions of cerebellar cortex Lobes Superior surface External Features Two deep fissures Primary fissure Posterosuperior fissure Three lobs Flocculonodular lobe 絨球小結葉 flocculus and nodule Anterior lobe -

The Cerebellum
2014/4/11 General View of Cerebellum 10 % total volume of the brain, but >50% of all its neurons The Cerebellum Provided with extensive information (40 times more axons project into the cerebellum than exit from it) Not necessary to basic elements of perception or movement. 陽明大學醫學院 腦科所 Damage to the cerebellum disrupts the spatial accuracy 陳昌明 副教授 and temporal coordination of movement. It impairs balance and reduces muscle tone and motor learning and certain cognitive functions. Position Lies above and behind the medullar and pons and occupies posterior cranial fossa Cerebellum Cerebellum external configuration • Located in posterior cranial fossa • Tentorium cerebelli (cerebrum), 4th ventricle (brain stem) • Communicate with other structure via •superior, middle, and inferior cerebellar peduncle 1 2014/4/11 External features External features Consists of two cerebellar hemisphere united in the Three peduncles midline by the vermis Inferior cerebellar peduncle 下小腦脚 -connect with medulla and with spinal cord, contain both afferent and efferent fibers Middle cerebellar peduncle 中小腦脚-connect with pons, contain afferent fibers Superior cerebellar peduncle 上小腦脚-connect with midbrain, contain mostly efferent fibers Cerebellum Lobes Anterior lobe corpus of Primary fissure cerebellar Longitudinal division Posterior lobe Vermis, Paravermal Region, Cerebellar Hemisphere Transverse division Flocculonodular lobe Anterior Lobe Posterolateral fissure Posterior Lobe Flocculonodular Lobe Lobes External features Superior surface Two deep fissures Primary fissure Tonsil of cerebellum 小腦扁桃体 two elevated Posterosuperior fissure masses on inferior Three lobs surface of hemispheral Flocculonodular lobe 絨球小結葉 portion just nearby flocculus and nodule foramen magnum Anterior lobe Corpus of cerebellar Posterior lobe Tonsil View from below 2 2014/4/11 External features Cerebellum & Brainstem, Inferior Surface, Anterior View Internal structures Deep Nuclei Gray matter Cerebellar cortex Cerebellar nuclei Dentate nucleus 齒狀核 1.