(Para)Flocculus Mennink, Lilian M; Van Dijk, J Marc C; Van Dijk, Pim
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

The Cerebellum in Sagittal Plane-Anatomic-MR Correlation: 2
667 The Cerebellum in Sagittal Plane-Anatomic-MR Correlation: 2. The Cerebellar Hemispheres Gary A. Press 1 Thin (5-mm) sagittal high-field (1 .5-T) MR images of the cerebellar hemispheres James Murakami2 display (1) the superior, middle, and inferior cerebellar peduncles; (2) the primary white Eric Courchesne2 matter branches to the hemispheric lobules including the central, anterior, and posterior Dean P. Berthoty1 quadrangular, superior and inferior semilunar, gracile, biventer, tonsil, and flocculus; Marjorie Grafe3 and (3) several finer secondary white-matter branches to individual folia within the lobules. Surface features of the hemispheres including the deeper fissures (e.g., hori Clayton A. Wiley3 1 zontal, posterolateral, inferior posterior, and inferior anterior) and shallower sulci are John R. Hesselink best delineated on T1-weighted (short TRfshort TE) and T2-weighted (long TR/Iong TE) sequences, which provide greatest contrast between CSF and parenchyma. Correlation of MR studies of three brain specimens and 11 normal volunteers with microtome sections of the anatomic specimens provides criteria for identifying confidently these structures on routine clinical MR. MR should be useful in identifying, localizing, and quantifying cerebellar disease in patients with clinical deficits. The major anatomic structures of the cerebellar vermis are described in a companion article [1). This communication discusses the topographic relationships of the cerebellar hemispheres as seen in the sagittal plane and correlates microtome sections with MR images. Materials, Subjects, and Methods The preparation of the anatomic specimens, MR equipment, specimen and normal volunteer scanning protocols, methods of identifying specific anatomic structures, and system of This article appears in the JulyI August 1989 issue of AJNR and the October 1989 issue of anatomic nomenclature are described in our companion article [1]. -

CONGENITAL ABNORMALITIES of the CENTRAL NERVOUS SYSTEM Christopher Verity, Helen Firth, Charles Ffrench-Constant *I3
J Neurol Neurosurg Psychiatry: first published as 10.1136/jnnp.74.suppl_1.i3 on 1 March 2003. Downloaded from CONGENITAL ABNORMALITIES OF THE CENTRAL NERVOUS SYSTEM Christopher Verity, Helen Firth, Charles ffrench-Constant *i3 J Neurol Neurosurg Psychiatry 2003;74(Suppl I):i3–i8 dvances in genetics and molecular biology have led to a better understanding of the control of central nervous system (CNS) development. It is possible to classify CNS abnormalities Aaccording to the developmental stages at which they occur, as is shown below. The careful assessment of patients with these abnormalities is important in order to provide an accurate prog- nosis and genetic counselling. c NORMAL DEVELOPMENT OF THE CNS Before we review the various abnormalities that can affect the CNS, a brief overview of the normal development of the CNS is appropriate. c Induction—After development of the three cell layers of the early embryo (ectoderm, mesoderm, and endoderm), the underlying mesoderm (the “inducer”) sends signals to a region of the ecto- derm (the “induced tissue”), instructing it to develop into neural tissue. c Neural tube formation—The neural ectoderm folds to form a tube, which runs for most of the length of the embryo. c Regionalisation and specification—Specification of different regions and individual cells within the neural tube occurs in both the rostral/caudal and dorsal/ventral axis. The three basic regions of copyright. the CNS (forebrain, midbrain, and hindbrain) develop at the rostral end of the tube, with the spinal cord more caudally. Within the developing spinal cord specification of the different popu- lations of neural precursors (neural crest, sensory neurones, interneurones, glial cells, and motor neurones) is observed in progressively more ventral locations. -

Neurologic Outcomes in Friedreich Ataxia: Study of a Single-Site Cohort E415
Volume 6, Number 3, June 2020 Neurology.org/NG A peer-reviewed clinical and translational neurology open access journal ARTICLE Neurologic outcomes in Friedreich ataxia: Study of a single-site cohort e415 ARTICLE Prevalence of RFC1-mediated spinocerebellar ataxia in a North American ataxia cohort e440 ARTICLE Mutations in the m-AAA proteases AFG3L2 and SPG7 are causing isolated dominant optic atrophy e428 ARTICLE Cerebral autosomal dominant arteriopathy with subcortical infarcts and leukoencephalopathy revisited: Genotype-phenotype correlations of all published cases e434 Academy Officers Neurology® is a registered trademark of the American Academy of Neurology (registration valid in the United States). James C. Stevens, MD, FAAN, President Neurology® Genetics (eISSN 2376-7839) is an open access journal published Orly Avitzur, MD, MBA, FAAN, President Elect online for the American Academy of Neurology, 201 Chicago Avenue, Ann H. Tilton, MD, FAAN, Vice President Minneapolis, MN 55415, by Wolters Kluwer Health, Inc. at 14700 Citicorp Drive, Bldg. 3, Hagerstown, MD 21742. Business offices are located at Two Carlayne E. Jackson, MD, FAAN, Secretary Commerce Square, 2001 Market Street, Philadelphia, PA 19103. Production offices are located at 351 West Camden Street, Baltimore, MD 21201-2436. Janis M. Miyasaki, MD, MEd, FRCPC, FAAN, Treasurer © 2020 American Academy of Neurology. Ralph L. Sacco, MD, MS, FAAN, Past President Neurology® Genetics is an official journal of the American Academy of Neurology. Journal website: Neurology.org/ng, AAN website: AAN.com CEO, American Academy of Neurology Copyright and Permission Information: Please go to the journal website (www.neurology.org/ng) and click the Permissions tab for the relevant Mary E. -

Defining, Diagnosing, Clarifying, and Classifying the Chiari I Malformations
Child's Nervous System (2019) 35:1785–1792 https://doi.org/10.1007/s00381-019-04172-6 SPECIAL ANNUAL ISSUE Defining, diagnosing, clarifying, and classifying the Chiari I malformations Stephen Bordes1,2 & Skyler Jenkins1,2 & R. Shane Tubbs1 Received: 3 April 2019 /Accepted: 21 April 2019 /Published online: 2 May 2019 # Springer-Verlag GmbH Germany, part of Springer Nature 2019 Abstract Purpose Chiari malformations (CM) have been traditionally classified into four categories: I, II, III, and IV. In light of more recent understandings, variations of the CM have required a modification of this classification. Methods This article discusses the presentation, diagnostics, and treatment of the newer forms of hindbrain herniation associated with the CM type I. Results The CM 1 is a spectrum that includes some patients who do not fall into the exact category of this entity. Conclusions While CM have been categorically recognized as discrete and individual conditions, newer classifications such as CM 0 and CM 1.5 exhibit some degree of continuity with CM 1; however, they require distinct and separate classification as symptoms and treatments can vary among these clinical subtypes. Keywords Chiari . Chiari 0 . Chiari 1.5 . Neurosurgery Abbreviations herniation of the cerebellar tonsil that extends inferiorly to the CM Chiari malformation foramen magnum. However, in light of more recent advance- CSF Cerebrospinal fluid ments in clinical medicine, Chiari 0 (Figs. 2 and 3) and 1.5 malformations (Fig. 4)havebeenrecognizedasvariationsof the Chiari I malformation. Chiari 0 malformations are consid- Introduction ered borderline defects in which a syringohydromyelia re- sponds to decompression of the posterior cranial fossa al- though there is little to no cerebellar tonsillar herniation (< Chiari malformations are structural variations of the cerebel- 3 mm). -

The Stellate Cells of the Rat's Cerebellar Cortex*
Z. Anat. Entwickl.-Gesch. 136, 224--248 (1972) by Springer-Verlag 1972 The Stellate Cells of the Rat's Cerebellar Cortex* Victoria Chan-Palay and Sanford L. Palay Departments of Neurobiology and Anatomy, Harvard Medical School, Boston, Massachusetts Received February 18, 1972 Summary. Stellate cells were studied in rapid Golgi preparations and in electron micro- graphs. These small neurons can be classified on the basis of their position in the molecular layer and the patterns of their dendritic and axonal arborizations as follows: (1) superficial cells with short, contorted dendrites and a circumscribed axonal arbor (upper third of the molecular layer) ; (2) deep stellate cells with radiating, twisted dendrites and with long axons giving rise to thin, varicose collaterals (middle third of the molecular layer); (3) deep stellate cells with similar dendrites and long axons giving collaterals to the basket around the Purkinje cell bodies (middle third of the molecular layer). An important characteristic of the stellate cell axon is that it generates most of its collaterals close to its origin. Even in long axon cells, only a few collaterals issue from the more distant parts of the axon. These forms contrast with the basket cell, which sends out long, straighter dendrites, and an extended axon that first emits branches at some distance from its origin. Furthermore, basket cell axon collaterals are usually stout in contrast to the frail, beaded collaterals of the stellate cell axon. The two cell types are considered to be distinct. In electron micrographs stellate cells display folded nuclei and sparse cytoplasm with the characteristics usual for small neurons. -

Tonsillar Herniation Extending More Than 5 Mm Below the Foramen Magnum on MR Imaging of the Head Is 0.78% (36)
Surgical Experience in Pediatric Patients with Chiari I malformations age ≤ 18 years Submitted for MCh Neurosurgery By Dr. Vipin Kumar October - 2012 Department of Neurosurgery Sree Chitra Tirunal Institute for Medical Sciences & Technology Thiruvananthapuram – 695011 1 Surgical Experience in Pediatric Patients with Chiari I malformations age ≤ 18 years Submitted by : Dr. Vipin Kumar Programme : MCh Neurosurgery Month & year of submission : October, 2012 2 CERTIFICATE This is to certify that the thesis entitled ―Surgical experience in pediatric patients with Chiari I malformations age ≤ 18 years‖ is a bonafide work of Dr. Vipin Kumar and was conducted in the Department of Neurosurgery, Sree Chitra Tirunal Institute for Medical Sciences & Technology, Thiruvananthapuram (SCTIMST), under my guidance and supervision. Dr. Suresh Nair Professor and Head Department of Neurosurgery SCTIMST, Thiruvananthapuram 3 DECLARATION This thesis titled “Surgical experience in pediatric patients with Chiari I malformations age ≤ 18 years ” is a consolidated report based on a bonafide study of the period from January 1999 to June 2011, done by me under the Department of Neurosurgery, Sree Chitra Tirunal Institute for Medical Sciences & Technology, Thiruvananthapuram. This thesis is submitted to SCTIMST in partial fulfillment of rules and regulations of MCh Neurosurgery examination. Dr. Vipin Kumar Department of Neurosurgery, SCTIMST, Thiruvananthapuram. 4 ACKNOWLEDGEMENT The guidance of Dr. Suresh Nair, Professor and Head of the Department of Neurosurgery, has been invaluable and I am extremely grateful and indebted for his contributions and suggestions, which were of invaluable help during the entire work. He will always be a constant source of inspiration to me. I owe a deep sense of gratitude to Dr. -
Homo Sapiens (445) MEDIUM (=IQR) HIGH
Dataset: 445 anatomical parts from data selection: HS_mRNASeq_HUMAN_GL-1 Showing 2 measure(s) of 2 gene(s) on selection: HS-0 TMPRSS2 ACE2 Level of expression Homo sapiens (445) MEDIUM (=IQR) HIGH 0 1 2 3 4 5 6 7 8 9 10 11 samples avg. expr. Tissue 3435 1.42 gestational structure 4 1.92 extraembryonic tissue / fluid 4 1.92 placenta 4 1.92 alimentary system 721 3.93 gastrointestinal tract 519 4.83 mouth (oral cavity) 7 0.87 oral mucosa (unspecified) 4 1.13 salivary gland 3 0.54 stomach 34 2.82 gastric (stomach) region 31 2.90 stomach cardia 11 2.13 stomach body 11 4.06 pyloric antrum 9 2.42 intestine 463 5.14 large intestine 294 3.48 caecum (cecum) 7 3.17 apex of caecum (appendix) 3 1.96 colorectum 287 3.49 colon 207 3.60 colonic mucosa 84 3.43 proximal colon 35 3.20 proximal colonic mucosa 35 3.20 ascending colon 30 3.32 transverse colon 5 2.53 distal colon 31 2.94 distal colonic mucosa 31 2.94 descending colon 6 2.51 sigmoid colon 25 3.05 rectosigmoid colon 5 2.33 rectosigmoid junction 6 3.47 rectum 62 3.00 small intestine 168 8.07 ileum 157 8.36 ileal mucosa 76 8.78 esophagus (oesophagus) 15 1.60 liver and biliary system 130 1.50 liver 119 1.36 gall bladder 3 6.94 bile duct 8 1.56 intrahepatic bile duct 8 1.56 pancreas 72 1.83 pancreatic islet (islet of Langerhans) 70 1.87 circulatory system 791 0.21 cardiovascular system 26 3.51 heart 19 3.39 heart muscle (myocardium, cardiac muscle) 7 4.10 heart ventricle 3 3.88 heart left ventricle 3 3.88 left ventricle free wall 3 3.88 vascular system 7 3.83 blood vessel 7 3.83 artery 7 3.83 coronary -

Tonsillobiventral Fissure Approach to the Lateral Recess of the Fourth Ventricle Ali Tayebi Meybodi, MD, Michael T
LABORATORY INVESTIGATION Tonsillobiventral fissure approach to the lateral recess of the fourth ventricle Ali Tayebi Meybodi, MD, Michael T. Lawton, MD, Halima Tabani, MD, and Arnau Benet, MD Skull Base and Cerebrovascular Laboratory, Department of Neurosurgery, University of California, San Francisco, California OBJECTIVE Surgical access to the lateral recess of the fourth ventricle (LR) is suboptimal with existing transvermian and telovelar approaches because of limited lateral exposure, significant retraction of the cerebellar tonsil, and steep trajectories near brainstem perforator arteries. The goal in this study was to assess surgical exposure of the tonsillobi- ventral fissure approach to the LR, and to describe the relevant anatomy. METHODS Two formaldehyde-fixed cerebella were used to study the anatomical relationships of the LR. Also, the ton- sillobiventral fissure approach was simulated in 8 specimens through a lateral suboccipital craniotomy. RESULTS The pattern of the cerebellar folia and the cortical branches of the posterior inferior cerebellar artery were key landmarks to identifying the tonsillobiventral fissure. Splitting the tonsillobiventral fissure allowed a direct and safe surgical trajectory to the LR and into the cerebellopontine cistern. The proposed approach reduces cervical flexion and optimizes the surgical angle of attack. CONCLUSIONS The tonsillobiventral fissure approach is a feasible and effective option for exposing the LR. This approach has more favorable trajectories and positions for the patient and the surgeon, and it should be added to the armamentarium for lesions in this location. https://thejns.org/doi/abs/10.3171/2016.8.JNS16855 KEY WORDS cerebellar tonsil; telovelar approach; cerebellomedullary fissure; inferior medullary velum; biventral lobule; tela choroidea; cerebellopontine cistern; vascular disorders; anatomy HE lateral recess of the fourth ventricle (LR) is a positioning and creating an uncomfortable position for the narrow space bounded by vital neural and vascular surgeon. -
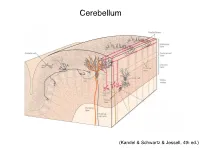
Cerebellum and Activation of the Cerebellum IO ST During Nonmotor Tasks
Cerebellum (Kandel & Schwartz & Jessell, 4th ed.) Granule186 cells are irreducibly smallChapter (6-8 7 μm) stellate cell basket cell outer synaptic layer PC rs cap PC layer grc Go inner 6 μm synaptic layer Figure 7.15 Largest cerebellar neuron occupies more than a 1,000-fold greater volume than smallest neuron. Thin section (~1 μ m) through monkey cerebellar cortex. Purkinje cell body (PC) and nucleus are far larger than those of granule cell (grc). The latter cluster to leave space for mossy fiber terminals to form glomeruli with grc dendritic claws and space for Golgi cells (Go). Note rich network of capillaries (cap). Fine, scat- tered dots are mitochondria. Courtesy of E. Mugnaini. brain ’ s most numerous neuron, this small cost grows large (see also chapter 13). Much of the inner synaptic layer is occupied by the large axon terminals of mossy fibers (figures 7.1 and 7.16). A terminal interlaces with multiple (~15) dendritic claws, each from a different but neighboring granule cell, and forms a complex knot (glomerulus ), nearly as large as a granule cell body (figures 7.1 and 7.16). The mossy fiber axon fires at an unusually high mean rate (up to 200 Hz) and is therefore among the brain’ s thickest (figure 4.6). To match the axon’ s high rate, a terminal expresses 150 active zones, 10 per postsynaptic granule cell (figure 7.16). These sites are capable of driving … and most expensive. 190 Chapter 7 10 4 8 3 9 19 10 10 × 6 × 2 2 4 ATP/s/cell ATP/s/cell ATP/s/m 1 2 0 0 astrocyte astrocyte Golgi cell Golgi cell basket cell basket cell stellate cell stellate cell granule cell granule cell mossy fiber mossy fiber Purkinje cell Purkinje Purkinje cell Purkinje Bergman glia Bergman glia climbing fiber climbing fiber Figure 7.18 Energy costs by cell type. -

The Modifiable Neuronal Network of the Cerebellum
Japanese Journal of Physiology, 34, 781-792, 1984 MINIREVIEW The Modifiable Neuronal Network of the Cerebellum M asao ITO Department of Physiology, Faculty of Medicine, University of Tokyo, Bunkyo-ku, Tokyo, 113 Japan An outstanding feature of the cerebellum known since the classic histological work of Ramon y Cajal is the relative simplicity and highly ordered geometry of its neuronal network. The cerebellar neuronal network consists of five major types of cortical neurons (Purkinje, basket, stellate, Golgi, and granule cells), two major types of afferents (mossy and climbing fibers), and the subcortical cells in the vestibular and cerebellar nuclei. When the neuronal network structure of the cerebellum was comprehensively dissected and described in great detail in the 1960s, and summarized by ECCLES,ITO, and SZ.ENTAGOTHAI[8], it was taken as a challenge to understand more completely the complex functions of the central nervous system using these fundamental studies of the brain as groundwork for future elucidation. As the gap between our understanding of the brain and our knowledge of neuronal networks is in general so large, hope has arisen that cere- bellar physiology may successfully fill the gap rather soon. Rigorous efforts using numerous newly-introduced techniques have been devoted during the past two decades, yielding remarkable progress that meets expectations at least to some extent. A number of problems remain to be solved, however, before we fully understand the cerebellum. It seems important at this point to review the major achievements of the past two decades in order to gain a clearer perspective of cerebellar physiology for the coming decade. -

Computed Tomography of the Brain Stem with Intrathecal Metrizamide. Part I: the Normal Brain Stem
Computed Tomography of the Brain Stem with Intrathecal Metrizamide. Part I: The Normal Brain Stem Michel E. Mawad 1 Detailed anatomy of the brain stem and cervicomedullary junction can be accurately A. John Silver demonstrated with metrizamide computed tomographic cisternography. Specifically. Sadek K. Hilal surface anatomy is unusually well outlined. Nine distinct and easily recognizable levels S. Ramaiah Ganti of section are described: four levels in the medulla, three in the pons, and two in the mesencephalon. Surface features of the brain stem, fine details in the floor of the fourth ventricle, cranial nerves, and vascular structures are shown and discussed. Reliably accurate imaging of the brain stem and cervicomedullary junction has now become available using high-resolution computed tomographic (CT) scan ning following intrathecal admini stration of metrizamide [1 -6]. The demonstration of surface features of the brain stem such as the ventral fissure, ventrolateral su lcus, pyramids, and olivary protuberance has become commonplace; suc h details have not been routinely demonstrable in the past. Many authors [1, 2] have already emphasized the value of metrizamide CT cisternography and its superiority to both angiography and pneumoencephalog raphy. These latter procedures rely on subtle displacement of vessels or distor tion of the air-filled fourth ventricle and posterior fossa cisterns. Compared with air, metrizamide spreads much more readily in th e entire subarachnoid space without the problem of meniscus formation or " air lock. " CT permits the sepa ration of the various collections of contrast agent and avoids th e superimposition of features encountered in nontomographic contrast studies. Improved visualization of the details of the brain stem by metrizamide CT has allowed the detection of subtle morphologic changes in the brain stem and subarachnoid space not previously appreciated. -

Gross and Neurosurgical Anatomy of the Cerebellar Tonsil Clinical Anatomy in Mavridis1*
Page 1 of 6 Critical review Gross and neurosurgical anatomy of the cerebellar tonsil Clinical Anatomy IN Mavridis1* Abstract anomalies such as arterial Discussion Introduction hypoplasticity, makes this structure The author has referenced one of its The primary purpose of this review potentially vulnerable to vascular own studies in this review. This was to focus on the gross anatomy, accidents (mainly infarcts) of the referenced study has been conducted in including morphometric inferior cerebellar arteries. A accordance with the Declaration of characteristics and arterial thorough understanding of the Helsinki (1964) and the protocols of vasculature, of the human cerebellar regional tonsillar anatomy, as well as these studies have been approved by tonsil. We also aimed to investigate its relations with the branches of the the relevant ethics committees related the neurosurgical anatomy of this posterior inferior cerebellar artery is to the institution in which they were clinically important structure of the of paramount significance for performed. human brain. neurosurgical interventions in this Discussion area. Gross anatomy The cerebellar tonsil is a rounded Morphology, locations and relations lobule on the undersurface of each Introduction The cerebellar tonsil is a rounded cerebellar hemisphere, continuous The human cerebellar tonsil is famous lobule on the undersurface of each medially with the uvula of the for its herniation through the foramen cerebellar hemisphere, continuous cerebellar vermis and superiorly with magnum either in congenital or in medially with the uvula of the the flocculonodular lobe. Arterial acquired conditions. Synonyms of the cerebellar vermis and superiorly with branches entering the tonsil originate ‘cerebellar tonsil’ include ‘tonsilla the flocculonodular lobe2.