Physiology of Cells in the Central Lobes of the Mormyrid Cerebellum
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

The Stellate Cells of the Rat's Cerebellar Cortex*
Z. Anat. Entwickl.-Gesch. 136, 224--248 (1972) by Springer-Verlag 1972 The Stellate Cells of the Rat's Cerebellar Cortex* Victoria Chan-Palay and Sanford L. Palay Departments of Neurobiology and Anatomy, Harvard Medical School, Boston, Massachusetts Received February 18, 1972 Summary. Stellate cells were studied in rapid Golgi preparations and in electron micro- graphs. These small neurons can be classified on the basis of their position in the molecular layer and the patterns of their dendritic and axonal arborizations as follows: (1) superficial cells with short, contorted dendrites and a circumscribed axonal arbor (upper third of the molecular layer) ; (2) deep stellate cells with radiating, twisted dendrites and with long axons giving rise to thin, varicose collaterals (middle third of the molecular layer); (3) deep stellate cells with similar dendrites and long axons giving collaterals to the basket around the Purkinje cell bodies (middle third of the molecular layer). An important characteristic of the stellate cell axon is that it generates most of its collaterals close to its origin. Even in long axon cells, only a few collaterals issue from the more distant parts of the axon. These forms contrast with the basket cell, which sends out long, straighter dendrites, and an extended axon that first emits branches at some distance from its origin. Furthermore, basket cell axon collaterals are usually stout in contrast to the frail, beaded collaterals of the stellate cell axon. The two cell types are considered to be distinct. In electron micrographs stellate cells display folded nuclei and sparse cytoplasm with the characteristics usual for small neurons. -
Homo Sapiens (445) MEDIUM (=IQR) HIGH
Dataset: 445 anatomical parts from data selection: HS_mRNASeq_HUMAN_GL-1 Showing 2 measure(s) of 2 gene(s) on selection: HS-0 TMPRSS2 ACE2 Level of expression Homo sapiens (445) MEDIUM (=IQR) HIGH 0 1 2 3 4 5 6 7 8 9 10 11 samples avg. expr. Tissue 3435 1.42 gestational structure 4 1.92 extraembryonic tissue / fluid 4 1.92 placenta 4 1.92 alimentary system 721 3.93 gastrointestinal tract 519 4.83 mouth (oral cavity) 7 0.87 oral mucosa (unspecified) 4 1.13 salivary gland 3 0.54 stomach 34 2.82 gastric (stomach) region 31 2.90 stomach cardia 11 2.13 stomach body 11 4.06 pyloric antrum 9 2.42 intestine 463 5.14 large intestine 294 3.48 caecum (cecum) 7 3.17 apex of caecum (appendix) 3 1.96 colorectum 287 3.49 colon 207 3.60 colonic mucosa 84 3.43 proximal colon 35 3.20 proximal colonic mucosa 35 3.20 ascending colon 30 3.32 transverse colon 5 2.53 distal colon 31 2.94 distal colonic mucosa 31 2.94 descending colon 6 2.51 sigmoid colon 25 3.05 rectosigmoid colon 5 2.33 rectosigmoid junction 6 3.47 rectum 62 3.00 small intestine 168 8.07 ileum 157 8.36 ileal mucosa 76 8.78 esophagus (oesophagus) 15 1.60 liver and biliary system 130 1.50 liver 119 1.36 gall bladder 3 6.94 bile duct 8 1.56 intrahepatic bile duct 8 1.56 pancreas 72 1.83 pancreatic islet (islet of Langerhans) 70 1.87 circulatory system 791 0.21 cardiovascular system 26 3.51 heart 19 3.39 heart muscle (myocardium, cardiac muscle) 7 4.10 heart ventricle 3 3.88 heart left ventricle 3 3.88 left ventricle free wall 3 3.88 vascular system 7 3.83 blood vessel 7 3.83 artery 7 3.83 coronary -
Cerebellum and Activation of the Cerebellum IO ST During Nonmotor Tasks
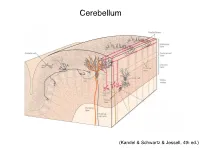
Cerebellum (Kandel & Schwartz & Jessell, 4th ed.) Granule186 cells are irreducibly smallChapter (6-8 7 μm) stellate cell basket cell outer synaptic layer PC rs cap PC layer grc Go inner 6 μm synaptic layer Figure 7.15 Largest cerebellar neuron occupies more than a 1,000-fold greater volume than smallest neuron. Thin section (~1 μ m) through monkey cerebellar cortex. Purkinje cell body (PC) and nucleus are far larger than those of granule cell (grc). The latter cluster to leave space for mossy fiber terminals to form glomeruli with grc dendritic claws and space for Golgi cells (Go). Note rich network of capillaries (cap). Fine, scat- tered dots are mitochondria. Courtesy of E. Mugnaini. brain ’ s most numerous neuron, this small cost grows large (see also chapter 13). Much of the inner synaptic layer is occupied by the large axon terminals of mossy fibers (figures 7.1 and 7.16). A terminal interlaces with multiple (~15) dendritic claws, each from a different but neighboring granule cell, and forms a complex knot (glomerulus ), nearly as large as a granule cell body (figures 7.1 and 7.16). The mossy fiber axon fires at an unusually high mean rate (up to 200 Hz) and is therefore among the brain’ s thickest (figure 4.6). To match the axon’ s high rate, a terminal expresses 150 active zones, 10 per postsynaptic granule cell (figure 7.16). These sites are capable of driving … and most expensive. 190 Chapter 7 10 4 8 3 9 19 10 10 × 6 × 2 2 4 ATP/s/cell ATP/s/cell ATP/s/m 1 2 0 0 astrocyte astrocyte Golgi cell Golgi cell basket cell basket cell stellate cell stellate cell granule cell granule cell mossy fiber mossy fiber Purkinje cell Purkinje Purkinje cell Purkinje Bergman glia Bergman glia climbing fiber climbing fiber Figure 7.18 Energy costs by cell type. -

The Modifiable Neuronal Network of the Cerebellum
Japanese Journal of Physiology, 34, 781-792, 1984 MINIREVIEW The Modifiable Neuronal Network of the Cerebellum M asao ITO Department of Physiology, Faculty of Medicine, University of Tokyo, Bunkyo-ku, Tokyo, 113 Japan An outstanding feature of the cerebellum known since the classic histological work of Ramon y Cajal is the relative simplicity and highly ordered geometry of its neuronal network. The cerebellar neuronal network consists of five major types of cortical neurons (Purkinje, basket, stellate, Golgi, and granule cells), two major types of afferents (mossy and climbing fibers), and the subcortical cells in the vestibular and cerebellar nuclei. When the neuronal network structure of the cerebellum was comprehensively dissected and described in great detail in the 1960s, and summarized by ECCLES,ITO, and SZ.ENTAGOTHAI[8], it was taken as a challenge to understand more completely the complex functions of the central nervous system using these fundamental studies of the brain as groundwork for future elucidation. As the gap between our understanding of the brain and our knowledge of neuronal networks is in general so large, hope has arisen that cere- bellar physiology may successfully fill the gap rather soon. Rigorous efforts using numerous newly-introduced techniques have been devoted during the past two decades, yielding remarkable progress that meets expectations at least to some extent. A number of problems remain to be solved, however, before we fully understand the cerebellum. It seems important at this point to review the major achievements of the past two decades in order to gain a clearer perspective of cerebellar physiology for the coming decade. -

Spontaneous Activity Signatures of Morphologically Identified Interneurons in the Vestibulocerebellum
712 • The Journal of Neuroscience, January 12, 2011 • 31(2):712–724 Behavioral/Systems/Cognitive Spontaneous Activity Signatures of Morphologically Identified Interneurons in the Vestibulocerebellum Tom J. H. Ruigrok,1 Robert A. Hensbroek,2 and John I. Simpson2 1Department of Neuroscience, Erasmus Medical Center Rotterdam, 3000 CA Rotterdam, The Netherlands, and 2Department of Physiology and Neuroscience, New York University School of Medicine, New York, New York 10016 Cerebellar cortical interneurons such as Golgi cells, basket cells, stellate cells, unipolar brush cells, and granule cells play an essential role in the operations of the cerebellum. However, detailed functional studies of the activity of these cells in both anesthetized and behaving animals have been hampered by problems in recognizing their physiological signatures. We have extracellularly recorded the spontane- ous activity of vestibulocerebellar interneurons in ketamine/xylazine-anesthetized rats and subsequently labeled them with Neurobiotin using the juxtacellular technique. After recovery and morphological identification of these cells, they were related to statistical measures of their spontaneous activity. Golgi cells display a somewhat irregular firing pattern with relatively low average frequencies. Unipolar brush cells are characterized by more regular firing at higher rates. Basket and stellate cells are alike in their firing characteristics, which mainly stand out by their irregularity; some of them are set apart by their very slow average rate. The spontaneous activity of interneurons examined in the ketamine/xylazine rabbit fit within this general pattern. In the rabbit, granule cells were identified by the spontaneous occurrence of extremely high-frequency bursts of action potentials, which were also recognized in the rat. -

Stellate Cell Computational Modeling Predicts Signal Filtering in the Molecular Layer Circuit of Cerebellum
www.nature.com/scientificreports OPEN Stellate cell computational modeling predicts signal fltering in the molecular layer circuit of cerebellum Martina Francesca Rizza1, Francesca Locatelli1, Stefano Masoli1, Diana Sánchez‑Ponce3, Alberto Muñoz3,4, Francesca Prestori1,5 & Egidio D’Angelo1,2,5* The functional properties of cerebellar stellate cells and the way they regulate molecular layer activity are still unclear. We have measured stellate cells electroresponsiveness and their activation by parallel fber bursts. Stellate cells showed intrinsic pacemaking, along with characteristic responses to depolarization and hyperpolarization, and showed a marked short‑term facilitation during repetitive parallel fber transmission. Spikes were emitted after a lag and only at high frequency, making stellate cells to operate as delay‑high‑pass flters. A detailed computational model summarizing these physiological properties allowed to explore diferent functional confgurations of the parallel fber—stellate cell—Purkinje cell circuit. Simulations showed that, following parallel fber stimulation, Purkinje cells almost linearly increased their response with input frequency, but such an increase was inhibited by stellate cells, which leveled the Purkinje cell gain curve to its 4 Hz value. When reciprocal inhibitory connections between stellate cells were activated, the control of stellate cells over Purkinje cell discharge was maintained only at very high frequencies. These simulations thus predict a new role for stellate cells, which could endow the molecular layer with low‑pass and band‑pass fltering properties regulating Purkinje cell gain and, along with this, also burst delay and the burst‑pause responses pattern. Te stellate cells (SCs) are inhibitory interneurons of the cerebellum molecular layer (ML) that were frst identi- fed in histological preparations by Golgi 1 and Cajal2,3. -

C-Kit Receptor and Ligand Expression in Postnatal Development of the Mouse Cerebellum Suggests a Function for C-Kif in Inhibitory Interneurons
The Journal of Neuroscience, December 1992, 72(12): 4663-4676 c-kit Receptor and Ligand Expression in Postnatal Development of the Mouse Cerebellum Suggests a Function for c-kif in Inhibitory Interneurons Katia Manova,1~2~a Rosemary F. Bachvarova,* Eric J. Huang,’ Sandra Sanchez,’ Stephen M. Pronovost,l Eric Velazquez,113 Barbara McGuire,*v3 and Peter Besmerl ‘Molecular Biology Department, Sloan Kettering Institute and Cornell University Graduate School of Medical Sciences, New York, New York 10021, *Department of Cell Biology and Anatomy, Cornell University Medical College, New York, New York 10021, and 3Laboratory of Neurobiology, Rockefeller University, New York, New York 10021 The c-kit receptor and its cognate ligand, KL, are encoded Increasing evidence implicates several tyrosine kinase receptor at the white spoffjng locus (IW) and the steel locus (9) of systems in neurogenic processes.NGF, brain-derived neuro- the mouse, respectively. S/and Wmutations affect the same trophic factor, and neurotrophin-3 were recently shown to be cellular targets in melanogenesis, gametogenesis and he- ligandsof the c-trk, c-trkB, and c-trkC tyrosine kinase receptors, matopoiesis during embryonic development and in adult life. respectively (Kaplan et al., 1991; Lamballe et al., 199 1; Squint0 c-kit is expressed in cellular targets of Wand SI mutations, et al., 1991). These factors regulate cell survival, differentiation, whereas KL is expressed in the microenvironment of these and neurotransmitter synthesisof distinct sets of neurons (for targets. c-kitand KL, however, are also expressed in tissues a review, seeBarde, 1989). In vitro, basicfibroblast growth factor and cell types that are not targets of W and Sl mutations, enhancesthe survival and differentiation of granule neurons including the brain. -

Subcortical Motor Systems: Cerebellum
NN 23 Outline Anatomy Subcortical Motor Cerebellar cortex Neuronal circuitry Systems: cerebellum Cerebellar connections 陽明大學醫學院 腦科所 Vestibulocerebellum Spinocerebellum 陳昌明 副教授 Neocerebellum Other cerebellar functions Motor Loops Pyramidal Tract and Associated Circuits Cortex ---> Subcortex ---> Cortex ---> Spinal cord upper motor neuron UMN Cerebellum coordination of movement Cerebellum BASAL Basal Ganglia GANGLIA pyramidal tract selection & initiation of voluntary movements ~ lower motor neuron UMN Cerebellar functions Cerebellum: Anatomy The main functions: Coordinating skilled voluntary movements by influencing muscle activity, Controlling equilibrium and muscle tone through connections with the vestibular system and the spinal cord and its gamma motor neurons. 1. Found in the posterior Cranial fossa 2. Forms a roof over the 4th ventricle 3. Lies above and behind the medullar and pons 1 NN 23 Cerebellum: Anatomy Cerebellum: External features Folia & lobules Longitudinal division analogous to gyrus Vermis, Paravermal Vermis - along midline Region, Cerebellar Hemisphere output ---> ventromedial pathway Transverse division Hemispheres Anterior Lobe Posterior Lobe output ---> lateral pathway Flocculonodular Deep cerebellar nuclei Lobe fastigial, interposed, & dentate Major output structures ~ Functional divisions of cerebellar cortex Lobes Superior surface External Features Two deep fissures Primary fissure Posterosuperior fissure Three lobs Flocculonodular lobe 絨球小結葉 flocculus and nodule Anterior lobe -

The Cerebellum
2014/4/11 General View of Cerebellum 10 % total volume of the brain, but >50% of all its neurons The Cerebellum Provided with extensive information (40 times more axons project into the cerebellum than exit from it) Not necessary to basic elements of perception or movement. 陽明大學醫學院 腦科所 Damage to the cerebellum disrupts the spatial accuracy 陳昌明 副教授 and temporal coordination of movement. It impairs balance and reduces muscle tone and motor learning and certain cognitive functions. Position Lies above and behind the medullar and pons and occupies posterior cranial fossa Cerebellum Cerebellum external configuration • Located in posterior cranial fossa • Tentorium cerebelli (cerebrum), 4th ventricle (brain stem) • Communicate with other structure via •superior, middle, and inferior cerebellar peduncle 1 2014/4/11 External features External features Consists of two cerebellar hemisphere united in the Three peduncles midline by the vermis Inferior cerebellar peduncle 下小腦脚 -connect with medulla and with spinal cord, contain both afferent and efferent fibers Middle cerebellar peduncle 中小腦脚-connect with pons, contain afferent fibers Superior cerebellar peduncle 上小腦脚-connect with midbrain, contain mostly efferent fibers Cerebellum Lobes Anterior lobe corpus of Primary fissure cerebellar Longitudinal division Posterior lobe Vermis, Paravermal Region, Cerebellar Hemisphere Transverse division Flocculonodular lobe Anterior Lobe Posterolateral fissure Posterior Lobe Flocculonodular Lobe Lobes External features Superior surface Two deep fissures Primary fissure Tonsil of cerebellum 小腦扁桃体 two elevated Posterosuperior fissure masses on inferior Three lobs surface of hemispheral Flocculonodular lobe 絨球小結葉 portion just nearby flocculus and nodule foramen magnum Anterior lobe Corpus of cerebellar Posterior lobe Tonsil View from below 2 2014/4/11 External features Cerebellum & Brainstem, Inferior Surface, Anterior View Internal structures Deep Nuclei Gray matter Cerebellar cortex Cerebellar nuclei Dentate nucleus 齒狀核 1. -

Generation of Cerebellar Interneurons from Dividing Progenitors in White Matter
View metadata, citation and similar papers at core.ac.uk brought to you by CORE provided by Elsevier - Publisher Connector Neuron, Vol. 16, 47±54, January, 1996, Copyright 1996 by Cell Press Generation of Cerebellar Interneurons from Dividing Progenitors in White Matter Lei Zhang and James E. Goldman derived from progenitors in the EGL (Altman, 1972; Ja- Department of Pathology cobson, 1991). Recent studies using quail-chick chime- The Center for Neurobiology and Behavior ras and transplantation of early postnatal EGL progeni- College of Physicians and Surgeons tors have countered this view, concluding that EGL cells Columbia University give rise exclusively to granule cells and, therefore, that New York, New York 10032 basket and stellate interneurons are derived from the primary germinal zone at the base of the cerebellum (Hallonet et al., 1990; Hallonet and Le Douarin, 1992; Gao and Hatten, 1994). Summary It has been unclear, however, how to reconcile this view with the cessation of proliferative activity in the The traditional view of the external granular layer of primary germinal zone by the time interneurons develop. the cerebellar cortex giving rise to interneurons has Although such proliferative activity has disappeared by been challenged by recent studies with quail-chick birth, many dividing cells reside in the cerebellar white chimeras. To clarify the time and site of origins of matter (WM) of young rodents (Uzman, 1960; Miale and interneurons, a retrovirus carrying the b-galactosi- Sidman, 1961; Fujita et al., 1966). These proliferating dase gene was injected into the deep cerebellar tissue cells have been thought to represent immature glia (Uz- or external granular layer of postnatal day 4/5 rats to man, 1960; Miale and Sidman, 1961; Fujita et al., 1966) label dividing progenitors. -
Neuroscience Research CLIMBING FIBERS (Excitatory Input) R&D Systems Off Ers a Wide Range of High Quality Products for Neuroscience Kv4 Research
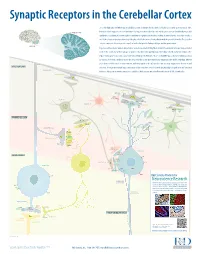
Synaptic Receptors in the Cerebellar Cortex The cerebellum (Latin for “little brain”) is a highly convoluted structure that lies at the level of the pons on the posterior aspect of the brainstem. It has long been associated with motor activity, and is believed to fi ne-tune muscle action associated with both posture and CEREBELLAR CORTEX equilibrium. In addition, it is now thought to contribute to cognitive and emotional activity. Anatomically, the cerebellum contains a well-defi ned outer cortex plus a distinct set of deeply-embedded neuron cell bodies that form the deep cerebellar nuclei. The erebellarc cortex is composed of three layers: the superfi cial molecular layer, the Purkinje cell layer, and the granular layer. CEREBELLUM In general, extracerebellar neurons project axons, termed mossy and climbing fi bers, to both the cerebellar cortex and deep cerebellar nuclei. In the cortex, mossy fi bers synapse on granule cells, which subsequently project to Purkinje cells, the dominant cell type in the region. Climbing fi bers, by contrast, project directly to Purkinje cells. Purkinje cells are strictly GABAergic, and provide inhibitory output to neurons of the deep cerebellar nuclei. The deep cerebellar nuclei represent the only output structure of the cerebellum. Eff erent axons from cerebellar nuclei neurons transmit inhibitory signals to the inferior olive and excitatory output to the brainstem and MOLECULAR LAYER thalamus. Although the multiple inputs and outputs of the cerebellum are well-defi ned, the physiological signifi cance and functional PARALLEL FIBERS relevance of these microcircuits remains to be established. R&D Systems currently off ers antibodies to all labeled molecules. -

(Para)Flocculus Mennink, Lilian M; Van Dijk, J Marc C; Van Dijk, Pim
View metadata, citation and similar papers at core.ac.uk brought to you by CORE provided by University of Groningen University of Groningen The cerebellar (para)flocculus Mennink, Lilian M; van Dijk, J Marc C; van Dijk, Pim Published in: Hearing Research DOI: 10.1016/j.heares.2020.108081 IMPORTANT NOTE: You are advised to consult the publisher's version (publisher's PDF) if you wish to cite from it. Please check the document version below. Document Version Publisher's PDF, also known as Version of record Publication date: 2020 Link to publication in University of Groningen/UMCG research database Citation for published version (APA): Mennink, L. M., van Dijk, J. M. C., & van Dijk, P. (2020). The cerebellar (para)flocculus: A review on its auditory function and a possible role in tinnitus. Hearing Research, 398, 108081. [108081]. https://doi.org/10.1016/j.heares.2020.108081 Copyright Other than for strictly personal use, it is not permitted to download or to forward/distribute the text or part of it without the consent of the author(s) and/or copyright holder(s), unless the work is under an open content license (like Creative Commons). Take-down policy If you believe that this document breaches copyright please contact us providing details, and we will remove access to the work immediately and investigate your claim. Downloaded from the University of Groningen/UMCG research database (Pure): http://www.rug.nl/research/portal. For technical reasons the number of authors shown on this cover page is limited to 10 maximum. Download date: 26-12-2020 Hearing Research 398 (2020) 108081 Contents lists available at ScienceDirect Hearing Research journal homepage: www.elsevier.com/locate/heares Review Article The cerebellar (para)flocculus: A review on its auditory function and a possible role in tinnitus ∗ Lilian M.