Generation of Cerebellar Interneurons from Dividing Progenitors in White Matter
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Plp-Positive Progenitor Cells Give Rise to Bergmann Glia in the Cerebellum
Citation: Cell Death and Disease (2013) 4, e546; doi:10.1038/cddis.2013.74 OPEN & 2013 Macmillan Publishers Limited All rights reserved 2041-4889/13 www.nature.com/cddis Olig2/Plp-positive progenitor cells give rise to Bergmann glia in the cerebellum S-H Chung1, F Guo2, P Jiang1, DE Pleasure2,3 and W Deng*,1,3,4 NG2 (nerve/glial antigen2)-expressing cells represent the largest population of postnatal progenitors in the central nervous system and have been classified as oligodendroglial progenitor cells, but the fate and function of these cells remain incompletely characterized. Previous studies have focused on characterizing these progenitors in the postnatal and adult subventricular zone and on analyzing the cellular and physiological properties of these cells in white and gray matter regions in the forebrain. In the present study, we examine the types of neural progeny generated by NG2 progenitors in the cerebellum by employing genetic fate mapping techniques using inducible Cre–Lox systems in vivo with two different mouse lines, the Plp-Cre-ERT2/Rosa26-EYFP and Olig2-Cre-ERT2/Rosa26-EYFP double-transgenic mice. Our data indicate that Olig2/Plp-positive NG2 cells display multipotential properties, primarily give rise to oligodendroglia but, surprisingly, also generate Bergmann glia, which are specialized glial cells in the cerebellum. The NG2 þ cells also give rise to astrocytes, but not neurons. In addition, we show that glutamate signaling is involved in distinct NG2 þ cell-fate/differentiation pathways and plays a role in the normal development of Bergmann glia. We also show an increase of cerebellar oligodendroglial lineage cells in response to hypoxic–ischemic injury, but the ability of NG2 þ cells to give rise to Bergmann glia and astrocytes remains unchanged. -

Spinal Cord Organization
Lecture 4 Spinal Cord Organization The spinal cord . Afferent tract • connects with spinal nerves, through afferent BRAIN neuron & efferent axons in spinal roots; reflex receptor interneuron • communicates with the brain, by means of cell ascending and descending pathways that body form tracts in spinal white matter; and white matter muscle • gives rise to spinal reflexes, pre-determined gray matter Efferent neuron by interneuronal circuits. Spinal Cord Section Gross anatomy of the spinal cord: The spinal cord is a cylinder of CNS. The spinal cord exhibits subtle cervical and lumbar (lumbosacral) enlargements produced by extra neurons in segments that innervate limbs. The region of spinal cord caudal to the lumbar enlargement is conus medullaris. Caudal to this, a terminal filament of (nonfunctional) glial tissue extends into the tail. terminal filament lumbar enlargement conus medullaris cervical enlargement A spinal cord segment = a portion of spinal cord that spinal ganglion gives rise to a pair (right & left) of spinal nerves. Each spinal dorsal nerve is attached to the spinal cord by means of dorsal and spinal ventral roots composed of rootlets. Spinal segments, spinal root (rootlets) nerve roots, and spinal nerves are all identified numerically by th region, e.g., 6 cervical (C6) spinal segment. ventral Sacral and caudal spinal roots (surrounding the conus root medullaris and terminal filament and streaming caudally to (rootlets) reach corresponding intervertebral foramina) collectively constitute the cauda equina. Both the spinal cord (CNS) and spinal roots (PNS) are enveloped by meninges within the vertebral canal. Spinal nerves (which are formed in intervertebral foramina) are covered by connective tissue (epineurium, perineurium, & endoneurium) rather than meninges. -
White Matter Tracts - Brain A143 (1)
WHITE MATTER TRACTS - BRAIN A143 (1) White Matter Tracts Last updated: August 8, 2020 CORTICOSPINAL TRACT .......................................................................................................................... 1 ANATOMY .............................................................................................................................................. 1 FUNCTION ............................................................................................................................................. 1 UNCINATE FASCICULUS ........................................................................................................................... 1 ANATOMY .............................................................................................................................................. 1 DTI PROTOCOL ...................................................................................................................................... 4 FUNCTION .............................................................................................................................................. 4 DEVELOPMENT ....................................................................................................................................... 4 CLINICAL SIGNIFICANCE ........................................................................................................................ 4 ARTICLES .............................................................................................................................................. -

Cerebellar Granule Cells in Culture
Proc. Nati. Acad. Sci. USA Vol. 83, pp. 4957-4961, July 1986 Neurobiology Cerebellar granule cells in culture: Monosynaptic connections with Purkinje cells and ionic currents (excitatory postsynaptic potential/patch-clamp) ToMoo HIRANO, YOSHIHIRo KUBO, AND MICHAEL M. WU Department of Neurobiology, Institute of Brain Research, School of Medicine, University of Tokyo, Tokyo, Japan Communicated by S. Hagiwara, March 6, 1986 ABSTRACT Electrophysiological properties of cerebellar tissue was dissociated by triturating with a fire-polished granule cells and synapses between granule and Purkinje cells Pasteur pipette in Ca-free Hanks' balanced salt solution were studied in dissociated cultures. Electrophysiological prop- containing 0.05% DNase and 12 mM MgSO4. The cells were erties of neurons and synapses in the mammalian central centrifuged at 150 x g at 40C and the pelleted cells were nervous system are best studied in dissociated cell cultures resuspended at a concentration of about 106 cells per ml in a because of good target cell visibility, control over the contents defined medium (9). One milliliter ofthe cell suspension from of the extracellular solution, and the feasibility of whole-cell newborn rats was plated first in a Petri dish (3.5 cm in patch electrode recording, which has been a powerful tech- diameter) containing several pieces ofheat-sterilized, poly(L- nique in analyzing biophysical properties of ionic channels in lysine)-coated coverslips, and then 1 ml of fetal cell suspen- small cells. We have applied this whole-cell recording technique sion was added. One-half of the culture medium was ex- to cultured cerebellar granule cells whose electrophysiological changed with fresh medium once a week. -

Memory Loss from a Subcortical White Matter Infarct
J Neurol Neurosurg Psychiatry: first published as 10.1136/jnnp.51.6.866 on 1 June 1988. Downloaded from Journal of Neurology, Neurosurgery, and Psychiatry 1988;51:866-869 Short report Memory loss from a subcortical white matter infarct CAROL A KOOISTRA, KENNETH M HEILMAN From the Department ofNeurology, College ofMedicine, University ofFlorida, and the Research Service, Veterans Administration Medical Center, Gainesville, FL, USA SUMMARY Clinical disorders of memory are believed to occur from the dysfunction of either the mesial temporal lobe, the mesial thalamus, or the basal forebrain. Fibre tract damage at the level of the fornix has only inconsistently produced amnesia. A patient is reported who suffered a cerebro- vascular accident involving the posterior limb of the left internal capsule that resulted in a persistent and severe disorder of verbal memory. The inferior extent of the lesion effectively disconnected the mesial thalamus from the amygdala and the frontal cortex by disrupting the ventral amygdalofugal and thalamic-frontal pathways as they course through the diencephalon. This case demonstrates that an isolated lesion may cause memory loss without involvement of traditional structures associated with memory and may explain memory disturbances in other white matter disease such as multiple sclerosis and lacunar state. Protected by copyright. Memory loss is currently believed to reflect grey day of his illness the patient was transferred to Shands matter damage of either the mesial temporal lobe,' -4 Teaching Hospital at the University of Florida for further the mesial or the basal forebrain.'0 l evaluation. thalamus,5-9 Examination at that time showed the patient to be awake, Cerebrovascular accidents resulting in memory dys- alert, attentive and fully oriented. -

White Matter Dissection and Structural Connectivity of the Human Vertical
www.nature.com/scientificreports OPEN White matter dissection and structural connectivity of the human vertical occipital fasciculus to link vision-associated brain cortex Tatsuya Jitsuishi1, Seiichiro Hirono2, Tatsuya Yamamoto1,3, Keiko Kitajo1, Yasuo Iwadate2 & Atsushi Yamaguchi1* The vertical occipital fasciculus (VOF) is an association fber tract coursing vertically at the posterolateral corner of the brain. It is re-evaluated as a major fber tract to link the dorsal and ventral visual stream. Although previous tractography studies showed the VOF’s cortical projections fall in the dorsal and ventral visual areas, the post-mortem dissection study for the validation remains limited. First, to validate the previous tractography data, we here performed the white matter dissection in post-mortem brains and demonstrated the VOF’s fber bundles coursing between the V3A/B areas and the posterior fusiform gyrus. Secondly, we analyzed the VOF’s structural connectivity with difusion tractography to link vision-associated cortical areas of the HCP MMP1.0 atlas, an updated map of the human cerebral cortex. Based on the criteria the VOF courses laterally to the inferior longitudinal fasciculus (ILF) and craniocaudally at the posterolateral corner of the brain, we reconstructed the VOF’s fber tracts and found the widespread projections to the visual cortex. These fndings could suggest a crucial role of VOF in integrating visual information to link the broad visual cortex as well as in connecting the dual visual stream. Te VOF is the fber tract that courses vertically at the posterolateral corner of the brain. Te VOF was histori- cally described in monkey by Wernicke1 and then in human by Obersteiner2. -
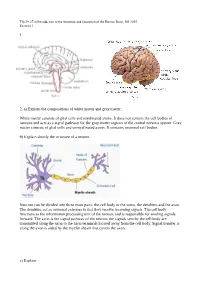
1. 2. A) Explain the Compositions of White Matter and Gray
Tfy-99.2710 Introduction to the Structure and Operation of the Human Brain, fall 2015 Exercise 1 1. 2. a) Explain the compositions of white matter and gray matter. White matter consists of glial cells and myelinated axons. It does not contain the cell bodies of neurons and acts as a signal pathway for the gray matter regions of the central nervous system. Gray matter consists of glial cells and unmyelinated axons. It contains neuronal cell bodies. b) Explain shortly the structure of a neuron. Neurons can be divided into three main parts: the cell body or the soma, the dendrites and the axon. The dendrites act as neuronal antennas in that they receive incoming signals. The cell body functions as the information processing unit of the neuron, and is responsible for sending signals forward. The axon is the signal pathway of the neuron; the signals sent by the cell body are transmitted along the axon to the axon terminals located away from the cell body. Signal transfer is along the axon is aided by the myelin sheath that covers the axon. c) Explain: Tfy-99.2710 Introduction to the Structure and Operation of the Human Brain, fall 2015 Exercise 1 i) myelin Membrane that wraps around axons. Formed from glial support cells: oligodendrogolia in the central nervous system and Schwann cells in the peripheral nervous system. Myelin facilitates signal transfer along axons by allowing action potentials to skip between the nodes of Ranvier (saltatory conduction). ii) receptor Molecule specialized in receiving a chemical signal by binding a specific neurotransmitter. -

NEUROGENESIS in the ADULT BRAIN: New Strategies for Central Nervous System Diseases
7 Jan 2004 14:25 AR AR204-PA44-17.tex AR204-PA44-17.sgm LaTeX2e(2002/01/18) P1: GCE 10.1146/annurev.pharmtox.44.101802.121631 Annu. Rev. Pharmacol. Toxicol. 2004. 44:399–421 doi: 10.1146/annurev.pharmtox.44.101802.121631 Copyright c 2004 by Annual Reviews. All rights reserved First published online as a Review in Advance on August 28, 2003 NEUROGENESIS IN THE ADULT BRAIN: New Strategies for Central Nervous System Diseases ,1 ,2 D. Chichung Lie, Hongjun Song, Sophia A. Colamarino,1 Guo-li Ming,2 and Fred H. Gage1 1Laboratory of Genetics, The Salk Institute, La Jolla, California 92037; email: [email protected], [email protected], [email protected] 2Institute for Cell Engineering, Department of Neurology, Johns Hopkins University School of Medicine, Baltimore, Maryland 21287; email: [email protected], [email protected] Key Words adult neural stem cells, regeneration, recruitment, cell replacement, therapy ■ Abstract New cells are continuously generated from immature proliferating cells throughout adulthood in many organs, thereby contributing to the integrity of the tissue under physiological conditions and to repair following injury. In contrast, repair mechanisms in the adult central nervous system (CNS) have long been thought to be very limited. However, recent findings have clearly demonstrated that in restricted areas of the mammalian brain, new functional neurons are constantly generated from neural stem cells throughout life. Moreover, stem cells with the potential to give rise to new neurons reside in many different regions of the adult CNS. These findings raise the possibility that endogenous neural stem cells can be mobilized to replace dying neurons in neurodegenerative diseases. -

The Stellate Cells of the Rat's Cerebellar Cortex*
Z. Anat. Entwickl.-Gesch. 136, 224--248 (1972) by Springer-Verlag 1972 The Stellate Cells of the Rat's Cerebellar Cortex* Victoria Chan-Palay and Sanford L. Palay Departments of Neurobiology and Anatomy, Harvard Medical School, Boston, Massachusetts Received February 18, 1972 Summary. Stellate cells were studied in rapid Golgi preparations and in electron micro- graphs. These small neurons can be classified on the basis of their position in the molecular layer and the patterns of their dendritic and axonal arborizations as follows: (1) superficial cells with short, contorted dendrites and a circumscribed axonal arbor (upper third of the molecular layer) ; (2) deep stellate cells with radiating, twisted dendrites and with long axons giving rise to thin, varicose collaterals (middle third of the molecular layer); (3) deep stellate cells with similar dendrites and long axons giving collaterals to the basket around the Purkinje cell bodies (middle third of the molecular layer). An important characteristic of the stellate cell axon is that it generates most of its collaterals close to its origin. Even in long axon cells, only a few collaterals issue from the more distant parts of the axon. These forms contrast with the basket cell, which sends out long, straighter dendrites, and an extended axon that first emits branches at some distance from its origin. Furthermore, basket cell axon collaterals are usually stout in contrast to the frail, beaded collaterals of the stellate cell axon. The two cell types are considered to be distinct. In electron micrographs stellate cells display folded nuclei and sparse cytoplasm with the characteristics usual for small neurons. -

Differences in the Developmental Patterns of Three Microtubule-Associated Proteins in the Rat Cerebellum’
0270.8474/85/0504-0977$02.00/O The Journal of Neuroscience Copyright 0 Society for Neuroscience Vol. 5, No. 4, pp. 977-991 Printed in U.S.A. April 1985 Differences in the Developmental Patterns of Three Microtubule-associated Proteins in the Rat Cerebellum’ ROBERT BERNHARDT,2 GERDA HUBER,3 AND ANDREW MATUS Friedrich Miescher-lnstitut, P. 0. Box 2543, CH-4002 Base/, Switzerland Abstract MAPS are potentially important physiological regulators of neuronal differentiation. The developmental distribution patternS of microtubule- It has become clear that microtubules of different tissues and cell associated proteins (MAPS) 1, 2, and 3 were studied using types are characterized by different MAPS. Thus, a category of high three monoclonal antibodies. lmmunochemical staining at the molecular weight MAPS, later identified as MAP2, is exclusively light and electron microscopic levels demonstrated the spe- expressed in adult neurons where it is most concentrated in den- cific localization of each MAP in different cellular and sub- drites (Matus et al., 1981, 1983; Caceres et al., 1983, 1984; Wiche cellular compartments. (i) MAPS, which is specifically asso- et al., 1983; Bernhardt and Matus, 1984; De Camilli et al., 1984). ciated with dendritic microtubules in the adult brain, is strictly This suggested the possibility that MAP2 could be specifically associated with growing dendrites from the onset of their involved in the formation of dendritic microtubules and hence could formation. (ii) MAP3, a recently described MAP of M, = play a significant role in the regulation of dendrite growth. This idea 180,000, which in the adult brain is associated with neurofi- found further support from immunohistochemical data of developing lament-rich axons and glial processes, is associated with brain (Bernhardt and Matus, 1982; Matus et al., 1983; Burgoyne and axons from the beginning of outgrowth. -

The Nomenclature of Human White Matter Association Pathways: Proposal for a Systematic Taxonomic Anatomical Classification
The Nomenclature of Human White Matter Association Pathways: Proposal for a Systematic Taxonomic Anatomical Classification Emmanuel Mandonnet, Silvio Sarubbo, Laurent Petit To cite this version: Emmanuel Mandonnet, Silvio Sarubbo, Laurent Petit. The Nomenclature of Human White Matter Association Pathways: Proposal for a Systematic Taxonomic Anatomical Classification. Frontiers in Neuroanatomy, Frontiers, 2018, 12, pp.94. 10.3389/fnana.2018.00094. hal-01929504 HAL Id: hal-01929504 https://hal.archives-ouvertes.fr/hal-01929504 Submitted on 21 Nov 2018 HAL is a multi-disciplinary open access L’archive ouverte pluridisciplinaire HAL, est archive for the deposit and dissemination of sci- destinée au dépôt et à la diffusion de documents entific research documents, whether they are pub- scientifiques de niveau recherche, publiés ou non, lished or not. The documents may come from émanant des établissements d’enseignement et de teaching and research institutions in France or recherche français ou étrangers, des laboratoires abroad, or from public or private research centers. publics ou privés. REVIEW published: 06 November 2018 doi: 10.3389/fnana.2018.00094 The Nomenclature of Human White Matter Association Pathways: Proposal for a Systematic Taxonomic Anatomical Classification Emmanuel Mandonnet 1* †, Silvio Sarubbo 2† and Laurent Petit 3* 1Department of Neurosurgery, Lariboisière Hospital, Paris, France, 2Division of Neurosurgery, Structural and Functional Connectivity Lab, Azienda Provinciale per i Servizi Sanitari (APSS), Trento, Italy, 3Groupe d’Imagerie Neurofonctionnelle, Institut des Maladies Neurodégénératives—UMR 5293, CNRS, CEA University of Bordeaux, Bordeaux, France The heterogeneity and complexity of white matter (WM) pathways of the human brain were discretely described by pioneers such as Willis, Stenon, Malpighi, Vieussens and Vicq d’Azyr up to the beginning of the 19th century. -
Homo Sapiens (445) MEDIUM (=IQR) HIGH
Dataset: 445 anatomical parts from data selection: HS_mRNASeq_HUMAN_GL-1 Showing 2 measure(s) of 2 gene(s) on selection: HS-0 TMPRSS2 ACE2 Level of expression Homo sapiens (445) MEDIUM (=IQR) HIGH 0 1 2 3 4 5 6 7 8 9 10 11 samples avg. expr. Tissue 3435 1.42 gestational structure 4 1.92 extraembryonic tissue / fluid 4 1.92 placenta 4 1.92 alimentary system 721 3.93 gastrointestinal tract 519 4.83 mouth (oral cavity) 7 0.87 oral mucosa (unspecified) 4 1.13 salivary gland 3 0.54 stomach 34 2.82 gastric (stomach) region 31 2.90 stomach cardia 11 2.13 stomach body 11 4.06 pyloric antrum 9 2.42 intestine 463 5.14 large intestine 294 3.48 caecum (cecum) 7 3.17 apex of caecum (appendix) 3 1.96 colorectum 287 3.49 colon 207 3.60 colonic mucosa 84 3.43 proximal colon 35 3.20 proximal colonic mucosa 35 3.20 ascending colon 30 3.32 transverse colon 5 2.53 distal colon 31 2.94 distal colonic mucosa 31 2.94 descending colon 6 2.51 sigmoid colon 25 3.05 rectosigmoid colon 5 2.33 rectosigmoid junction 6 3.47 rectum 62 3.00 small intestine 168 8.07 ileum 157 8.36 ileal mucosa 76 8.78 esophagus (oesophagus) 15 1.60 liver and biliary system 130 1.50 liver 119 1.36 gall bladder 3 6.94 bile duct 8 1.56 intrahepatic bile duct 8 1.56 pancreas 72 1.83 pancreatic islet (islet of Langerhans) 70 1.87 circulatory system 791 0.21 cardiovascular system 26 3.51 heart 19 3.39 heart muscle (myocardium, cardiac muscle) 7 4.10 heart ventricle 3 3.88 heart left ventricle 3 3.88 left ventricle free wall 3 3.88 vascular system 7 3.83 blood vessel 7 3.83 artery 7 3.83 coronary