The Perplexity Surrounding Chiari Malformations – Are We Any Wiser Now?
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-
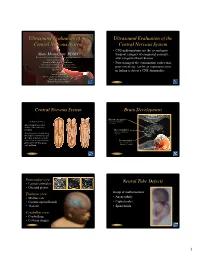
Ultrasound Evaluation of the Central Nervous System
Ultrasound Evaluation of the Ultrasound Evaluation of the Central Nervous System Central Nervous System ••CNSCNS malformations are the second most Mani Montazemi, RDMS frequent category of congenital anomaly, Director of Ultrasound Education & Quality Assurancee after congenital heart disease Baylor College of Medicine Division of Maternal-Fetal Medicine ••PoorPoor timing of the examination, rather than Department of Obstetrics and Gynecology Texas Children’s Hospital, Pavilion for Women poor sensitivity, can be an important factor Houston Texas & in failing to detect a CNS abnormality Clinical Instructor Thomas Jefferson University Hospital Radiology Department Fetal Head Philadelphia, Pennsylvania Fetal Head Central Nervous System Brain Development 9 -13 weeks Rhombencephalon 5th Menstrual Week •Gives rise to hindbrain •4th ventricle Arises from the posterior surface of the embryonic ectoderm Mesencephalon •Gives rise to midbrain A small groove is found along •Aqueduct the midline of the embryo and the edges of this groove fold over to form a neuro tube that Prosencephalon gives rise to the fetal spinal •Gives rise to forebrain rd cord and brain •Lateral & 3 ventricles Fetal Head Fetal Head Ventricular view Neural Tube Defects ••LateralLateral ventricles ••ChoroidChoroid plexus Group of malformations: Thalamic view • Anencephaly ••MidlineMidline falx •Anencephaly ••CavumCavum septiseptipellucidi pellucidi ••CephalocelesCephaloceles ••ThalamiThalami ••SpinaSpina bifida Cerebellar view ••CerebellumCerebellum ••CisternaCisterna magna Fetal -

The Chiari Malformations *
J Neurol Neurosurg Psychiatry: first published as 10.1136/jnnp.72.suppl_2.ii38 on 1 June 2002. Downloaded from THE CHIARI MALFORMATIONS Donald M Hadley ii38* J Neurol Neurosurg Psychiatry 2002;72(Suppl II):ii38–ii40 r Hans Chiari1 first described three hindbrain disorders associated with hydrocephalus in 1891. They have neither an anatomical nor embryological correlation with each other, but Dthey all involve the cerebellum and spinal cord and are thought to belong to the group of abnormalities that result from failure of normal dorsal induction. These include neural tube defects, cephaloceles, and spinal dysraphic abnormalities. Symptoms range from headache, sensory changes, vertigo, limb weakness, ataxia and imbalance to hearing loss. Only those with a type I Chiari malformation may be born grossly normal. The abnormalities are best shown on midline sagittal T1 weighted magnetic resonance imaging (MRI),2 but suspicious features on routine axial computed tomographic brain scans (an abnormal IVth ventricle, a “full” foramen magnum, and absent cisterna magna) should be recognised and followed up with MRI. c CHIARI I This is the mildest of the hindbrain malformations and is characterised by displacement of deformed cerebellar tonsils more than 5 mm caudally through the foramen magnum.3 The brain- stem and IVth ventricle retain a relatively normal position although the IVth ventricle may be small copyright. and slightly distorted (fig 1). A number of subgroups have been defined. c In the first group, intrauterine hydrocephalus causes tonsillar herniation. Once myelinated the tonsils retain this pointed configuration and ectopic position. Patients tend to present in child- hood with hydrocephalus and usually with syringomyelia. -

The Cerebellum in Sagittal Plane-Anatomic-MR Correlation: 2
667 The Cerebellum in Sagittal Plane-Anatomic-MR Correlation: 2. The Cerebellar Hemispheres Gary A. Press 1 Thin (5-mm) sagittal high-field (1 .5-T) MR images of the cerebellar hemispheres James Murakami2 display (1) the superior, middle, and inferior cerebellar peduncles; (2) the primary white Eric Courchesne2 matter branches to the hemispheric lobules including the central, anterior, and posterior Dean P. Berthoty1 quadrangular, superior and inferior semilunar, gracile, biventer, tonsil, and flocculus; Marjorie Grafe3 and (3) several finer secondary white-matter branches to individual folia within the lobules. Surface features of the hemispheres including the deeper fissures (e.g., hori Clayton A. Wiley3 1 zontal, posterolateral, inferior posterior, and inferior anterior) and shallower sulci are John R. Hesselink best delineated on T1-weighted (short TRfshort TE) and T2-weighted (long TR/Iong TE) sequences, which provide greatest contrast between CSF and parenchyma. Correlation of MR studies of three brain specimens and 11 normal volunteers with microtome sections of the anatomic specimens provides criteria for identifying confidently these structures on routine clinical MR. MR should be useful in identifying, localizing, and quantifying cerebellar disease in patients with clinical deficits. The major anatomic structures of the cerebellar vermis are described in a companion article [1). This communication discusses the topographic relationships of the cerebellar hemispheres as seen in the sagittal plane and correlates microtome sections with MR images. Materials, Subjects, and Methods The preparation of the anatomic specimens, MR equipment, specimen and normal volunteer scanning protocols, methods of identifying specific anatomic structures, and system of This article appears in the JulyI August 1989 issue of AJNR and the October 1989 issue of anatomic nomenclature are described in our companion article [1]. -

A Anencephaly
Glossary of Birth Anomaly Terms: A Anencephaly: A deadly birth anomaly where most of the brain and skull did not form. Anomaly: Any part of the body or chromosomes that has an unusual or irregular structure. Aortic valve stenosis: The aortic valve controls the flow of blood from the left ventricle of the heart to the aorta, which takes the blood to the rest of the body. If there is stenosis of this valve, the valve has space for blood to flow through, but it is too narrow. Atresia: Lack of an opening where there should be one. Atrial septal defect: An opening in the wall (septum) that separates the left and right top chambers (atria) of the heart. A hole can vary in size and may close on its own or may require surgery. Atrioventricular septal defect (endocardial cushion defect): A defect in both the lower portion of the atrial septum and the upper portion of the ventricular septum. Together, these defects make a large opening (canal) in the middle part of the heart. Aniridia (an-i-rid-e-a): An eye anomaly where the colored part of the eye (called the iris) is partly or totally missing. It usually affects both eyes. Other parts of the eye can also be formed incorrectly. The effects on children’s ability to see can range from mild problems to blindness. To learn more about aniridia, go to the U.S. National Library of Medicine website. Anophthalmia/microphthalmia (an-oph-thal-mia/mi-croph-thal-mia): Birth anomalies of the eyes. In anophthalmia, a baby is born without one or both eyes. -

Chiari Malformation by Ryan W Y Lee MD (Dr
Chiari malformation By Ryan W Y Lee MD (Dr. Lee of Shriners Hospitals for Children in Honolulu and the John A Burns School of Medicine at the University of Hawaii has no relevant financial relationships to disclose.) Originally released August 8, 1994; last updated March 9, 2017; expires March 9, 2020 Introduction This article includes discussion of Chiari malformation, Arnold-Chiari deformity, and Arnold-Chiari malformation. The foregoing terms may include synonyms, similar disorders, variations in usage, and abbreviations. Overview Chiari malformation describes a group of structural defects of the cerebellum, characterized by brain tissue protruding into the spinal canal. Chiari malformations are often associated with myelomeningocele, hydrocephalus, syringomyelia, and tethered cord syndrome. Although studies of etiology are few, an increasing number of specific genetic syndromes are found to be associated with Chiari malformations. Management primarily targets supportive care and neurosurgical intervention when necessary. Renewed effort to address current deficits in Chiari research involves work groups targeted at pathophysiology, symptoms and diagnosis, engineering and imaging analysis, treatment, pediatric issues, and related conditions. In this article, the author discusses the many aspects of diagnosis and management of Chiari malformation. Key points • Chiari malformation describes a group of structural defects of the cerebellum, characterized by brain tissue protruding into the spinal canal. • Chiari malformations are often associated -

Pushing the Limits of Prenatal Ultrasound: a Case of Dorsal Dermal Sinus Associated with an Overt Arnold–Chiari Malformation and a 3Q Duplication
reproductive medicine Case Report Pushing the Limits of Prenatal Ultrasound: A Case of Dorsal Dermal Sinus Associated with an Overt Arnold–Chiari Malformation and a 3q Duplication Olivier Leroij 1, Lennart Van der Veeken 2,*, Bettina Blaumeiser 3 and Katrien Janssens 3 1 Faculty of Medicine, University of Antwerp, 2610 Wilrijk, Belgium; [email protected] 2 Department of Obstetrics and Gynaecology, University Hospital Antwerp, 2650 Edegem, Belgium 3 Department of Medical Genetics, University Hospital and University of Antwerp, 2650 Edegem, Belgium; [email protected] (B.B.); [email protected] (K.J.) * Correspondence: [email protected] Abstract: We present a case of a fetus with cranial abnormalities typical of open spina bifida but with an intact spine shown on both ultrasound and fetal MRI. Expert ultrasound examination revealed a very small tract between the spine and the skin, and a postmortem examination confirmed the diagnosis of a dorsal dermal sinus. Genetic analysis found a mosaic 3q23q27 duplication in the form of a marker chromosome. This case emphasizes that meticulous prenatal ultrasound examination has the potential to diagnose even closed subtypes of neural tube defects. Furthermore, with cerebral anomalies suggesting a spina bifida, other imaging techniques together with genetic tests and measurement of alpha-fetoprotein in the amniotic fluid should be performed. Citation: Leroij, O.; Van der Veeken, Keywords: dorsal dermal sinus; Arnold–Chiari anomaly; 3q23q27 duplication; mosaic; marker chro- L.; Blaumeiser, B.; Janssens, K. mosome Pushing the Limits of Prenatal Ultrasound: A Case of Dorsal Dermal Sinus Associated with an Overt Arnold–Chiari Malformation and a 3q 1. -

Chiari Malformation and Hydrocephalus Masking Neurocysticercosis Sharad Rajpal1, Colson Tomberlin2, Andrew Bauer1, Robert C
Case Report Author's Personal Copy Chiari Malformation and Hydrocephalus Masking Neurocysticercosis Sharad Rajpal1, Colson Tomberlin2, Andrew Bauer1, Robert C. Forsythe3, Sigita Burneikiene1,4 Key words - BACKGROUND: Various diagnostic characteristics associated with neuro- - Chiari malformation cysticercosis have been well studied; however, their potential to be implicated - Hydrocephalus - Neurocysticercosis in other differential diagnoses has not been well demonstrated. - Subarachnoid cysts - CASE DESCRIPTION: We report the case of a 55-year-old Hispanic man who Abbreviations and Acronyms underwent a Chiari decompression surgery, which was complicated with hy- CP: Cerebellopontine drocephalus. Despite a ventriculoperitoneal shunt placement, he continued to MRI: Magnetic resonance imaging have headaches and was soon found to have several skull base subarachnoid VP: Ventriculoperitoneal lesions, which were later diagnosed as the sequelae of an active neuro- From the 1Boulder Neurosurgical Associates, 2University of cysticercosis infection. Colorado Boulder, 3Bouder Valley Pathology, and 4Justin Parker Neurological Institute, Boulder, Colorado, USA - CONCLUSION: This case report highlights the importance of overlapping To whom correspondence should be addressed: symptoms between diseases in a short temporal context. Sharad Rajpal, M.D. [E-mail: [email protected]] Citation: World Neurosurg. (2018) 114:68-71. https://doi.org/10.1016/j.wneu.2018.03.010 duraplasty. He had an uneventful hospital After approximately 8 months, the pa- Journal homepage: www.WORLDNEUROSURGERY.org course and was discharged home after 3 tient was seen in the emergency depart- days. The patient did well for several ment again for a fever, headache, balance Available online: www.sciencedirect.com months but then presented with recurrent problems, and myalgias. MRI of the brain 1878-8750/$ - see front matter ª 2018 Elsevier Inc. -

Auditory and Vestibular Systems Objective • to Learn the Functional
Auditory and Vestibular Systems Objective • To learn the functional organization of the auditory and vestibular systems • To understand how one can use changes in auditory function following injury to localize the site of a lesion • To begin to learn the vestibular pathways, as a prelude to studying motor pathways controlling balance in a later lab. Ch 7 Key Figs: 7-1; 7-2; 7-4; 7-5 Clinical Case #2 Hearing loss and dizziness; CC4-1 Self evaluation • Be able to identify all structures listed in key terms and describe briefly their principal functions • Use neuroanatomy on the web to test your understanding ************************************************************************************** List of media F-5 Vestibular efferent connections The first order neurons of the vestibular system are bipolar cells whose cell bodies are located in the vestibular ganglion in the internal ear (NTA Fig. 7-3). The distal processes of these cells contact the receptor hair cells located within the ampulae of the semicircular canals and the utricle and saccule. The central processes of the bipolar cells constitute the vestibular portion of the vestibulocochlear (VIIIth cranial) nerve. Most of these primary vestibular afferents enter the ipsilateral brain stem inferior to the inferior cerebellar peduncle to terminate in the vestibular nuclear complex, which is located in the medulla and caudal pons. The vestibular nuclear complex (NTA Figs, 7-2, 7-3), which lies in the floor of the fourth ventricle, contains four nuclei: 1) the superior vestibular nucleus; 2) the inferior vestibular nucleus; 3) the lateral vestibular nucleus; and 4) the medial vestibular nucleus. Vestibular nuclei give rise to secondary fibers that project to the cerebellum, certain motor cranial nerve nuclei, the reticular formation, all spinal levels, and the thalamus. -

CONGENITAL ABNORMALITIES of the CENTRAL NERVOUS SYSTEM Christopher Verity, Helen Firth, Charles Ffrench-Constant *I3
J Neurol Neurosurg Psychiatry: first published as 10.1136/jnnp.74.suppl_1.i3 on 1 March 2003. Downloaded from CONGENITAL ABNORMALITIES OF THE CENTRAL NERVOUS SYSTEM Christopher Verity, Helen Firth, Charles ffrench-Constant *i3 J Neurol Neurosurg Psychiatry 2003;74(Suppl I):i3–i8 dvances in genetics and molecular biology have led to a better understanding of the control of central nervous system (CNS) development. It is possible to classify CNS abnormalities Aaccording to the developmental stages at which they occur, as is shown below. The careful assessment of patients with these abnormalities is important in order to provide an accurate prog- nosis and genetic counselling. c NORMAL DEVELOPMENT OF THE CNS Before we review the various abnormalities that can affect the CNS, a brief overview of the normal development of the CNS is appropriate. c Induction—After development of the three cell layers of the early embryo (ectoderm, mesoderm, and endoderm), the underlying mesoderm (the “inducer”) sends signals to a region of the ecto- derm (the “induced tissue”), instructing it to develop into neural tissue. c Neural tube formation—The neural ectoderm folds to form a tube, which runs for most of the length of the embryo. c Regionalisation and specification—Specification of different regions and individual cells within the neural tube occurs in both the rostral/caudal and dorsal/ventral axis. The three basic regions of copyright. the CNS (forebrain, midbrain, and hindbrain) develop at the rostral end of the tube, with the spinal cord more caudally. Within the developing spinal cord specification of the different popu- lations of neural precursors (neural crest, sensory neurones, interneurones, glial cells, and motor neurones) is observed in progressively more ventral locations. -

Chiari Type II Malformation: Past, Present, and Future
Neurosurg Focus 16 (2):Article 5, 2004, Click here to return to Table of Contents Chiari Type II malformation: past, present, and future KEVIN L. STEVENSON, M.D. Children’s Healthcare of Atlanta, Atlanta, Georgia Object. The Chiari Type II malformation (CM II) is a unique hindbrain herniation found only in patients with myelomeningocele and is the leading cause of death in these individuals younger than 2 years of age. Several theories exist as to its embryological evolution and recently new theories are emerging as to its treatment and possible preven- tion. A thorough understanding of the embryology, anatomy, symptomatology, and surgical treatment is necessary to care optimally for children with myelomeningocele and prevent significant morbidity and mortality. Methods. A review of the literature was used to summarize the clinically pertinent features of the CM II, with par- ticular attention to pitfalls in diagnosis and surgical treatment. Conclusions. Any child with CM II can present as a neurosurgical emergency. Expeditious and knowledgeable eval- uation and prompt surgical decompression of the hindbrain can prevent serious morbidity and mortality in the patient with myelomeningocele, especially those younger than 2 years old. Symptomatic CM II in the older child often pre- sents with more subtle findings but rarely in acute crisis. Understanding of CM II continues to change as innovative techniques are applied to this challenging patient population. KEY WORDS • Chiari Type II malformation • myelomeningocele • pediatric The CM II is uniquely associated with myelomeningo- four distinct forms of the malformation, including the cele and is found only in this population. Originally de- Type II malformation that he found exclusively in patients scribed by Hans Chiari in 1891, symptomatic CM II ac- with myelomeningocele. -

NERVOUS SYSTEM هذا الملف لالستزادة واثراء المعلومات Neuropsychiatry Block
NERVOUS SYSTEM هذا الملف لﻻستزادة واثراء المعلومات Neuropsychiatry block. قال تعالى: ) َو َل َق د َخ َل قنَا ا ِْلن َسا َن ِمن ُس ََل َل ة ِ من ِطي ن }12{ ثُ م َجعَ لنَاه ُ نُ ط َفة فِي َق َرا ر م ِكي ن }13{ ثُ م َخ َل قنَا ال ُّن ط َفة َ َع َل َقة َف َخ َل قنَا ا لعَ َل َقة َ ُم ضغَة َف َخ َل قنَا ا ل ُم ضغَة َ ِع َظا ما َف َك َس ونَا ا ل ِع َظا َم َل ح ما ثُ م أَن َشأنَاه ُ َخ ل قا آ َخ َر َفتَبَا َر َك ّللا ُ أَ ح َس ُن ا ل َخا ِل ِقي َن }14{( Resources BRS Embryology Book. Pathoma Book ( IN DEVELOPMENTAL ANOMALIES PART ). [email protected] 1 OVERVIEW A- Central nervous system (CNS) is formed in week 3 of development, during which time the neural plate develops. The neural plate, consisting of neuroectoderm, becomes the neural tube, which gives rise to the brain and spinal cord. B- Peripheral nervous system (PNS) is derived from three sources: 1. Neural crest cells 2. Neural tube, which gives rise to all preganglionic autonomic nerves (sympathetic and parasympathetic) and all nerves (-motoneurons and -motoneurons) that innervate skeletal muscles 3. Mesoderm, which gives rise to the dura mater and to connective tissue investments of peripheral nerve fibers (endoneurium, perineurium, and epineurium) DEVELOPMENT OF THE NEURAL TUBE Neurulation refers to the formation and closure of the neural tube. BMP-4 (bone morphogenetic protein), noggin (an inductor protein), chordin (an inductor protein), FGF-8 (fibroblast growth factor), and N-CAM (neural cell adhesion molecule) appear to play a role in neurulation. -

12 Neurological Anomalies
BIOL 6505 − INTRODUCTION TO FETAL MEDICINE 12. NEUROLOGICAL ANOMALIES Petra Klinge, M.D. TOPICS • Myelodysplasia - Open vs. Closed neural tube defects • Hydrocephalus Congenital vs. Acquired I. MYELOMENINGOCELE (MMC)/OPEN NEURAL TUBE DEFECT • Single most common congenital defect of the central nervous system • 4.5/10,000 live births • 1500 cases/year despite dietary folate supplementation (50% reduction) • $200,000,000 health care dollars/year A. MMC - Embryogenesis • Initial closure of neural tube - day 21-23 • Cranial neuropore closure - day 23-25 • Caudal neuropore closure - day 25-27 • Spinal occlusion and initial ventricular expansion - day 25-32 • Secondary Neurulation - Caudal cell mass, Cavitation/retrogressive differentiation - day 27-54 B. Unified Mechanism D.G. McLone, M.D., Ph.D. • Open neural tube defect and leak of CSF into amniotic fluid - AFP positive • Loss of IV ventricular dilatation and expansion of rhombencephalon/posterior fossa • Small posterior fossa and creation of Chiari II malformation (Arnold Chiari malformation) C. MMC • Open neural tube defect - exposed neural placode • Chiari II malformation - Tectal beak, descent of IV ventricle, vermis herniation, medullary kink • Hydrocephalus, 85% require VPS D. MMC - Surgical Principals • Closure of open defect/MMC - 24-72 hours, minimizing risk of meningitis • CSF shunt/diversion for control of hydrocephalus, 80-90% cases 1 BIOL 6505 Spinal Dysraphism and Hydrocephalus: Neurosurgery in the Neonate (Continuation of Surgical Principals) • Chiari II decompression for stridor, airway obstruction, vocal cord paresis (less than 20%) E. Closure of MMC • Start IV antibiotics after birth • Cover neural placode with moist telfa and plastic wrap (saran). Keep moist • Avoid pressure to back. No peeking! • Family discussion with true objective • Planned, elective surgical procedure F.