Localization of Arabinogalactan Proteins in the Cell Walls of Developing Ovule in Strawberry (Fragaria X Ananassaduch.)
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Ap09 Biology Form B Q2
AP® BIOLOGY 2009 SCORING GUIDELINES (Form B) Question 2 Discuss the patterns of sexual reproduction in plants. Compare and contrast reproduction in nonvascular plants with that in flowering plants. Include the following topics in your discussion: (a) alternation of generations (b) mechanisms that bring female and male gametes together (c) mechanisms that disperse offspring to new locations Four points per part. Student must write about all three parts for full credit. Within each part it is possible to get points for comparing and contrasting. Also, specific points are available from details provided about nonvascular and flowering plants. Discuss the patterns of sexual reproduction in plants (4 points maximum): (a) Alternation of generations (4 points maximum): Topic Description (1 point each) Alternating generations Haploid stage and diploid stage. Gametophyte Haploid-producing gametes. Dominant in nonvascular plants. Double fertilization in flowering plants. Gametangia; archegonia and antheridia in nonvascular plants. Sporophyte Diploid-producing spores. Heterosporous in flowering plants. Flowering plants produce seeds; nonvascular plants do not. Flowering plants produce flower structures. Sporangia (megasporangia and microsporangia). Dominant in flowering plants. (b) Mechanisms that bring female and male gametes together (4 points maximum): Nonvascular Plants (1 point each) Flowering Plants (1 point each) Aquatic—requires water for motile sperm Terrestrial—pollination by wind, water, or animal Micropyle in ovule for pollen tube to enter Pollen tube to carry sperm nuclei Self- or cross-pollination Antheridia produce sperm Gametophytes; no antheridia or archegonia Archegonia produce egg Ovules produce female gametophytes/gametes Pollen: male gametophyte that produces gametes © 2009 The College Board. All rights reserved. Visit the College Board on the Web: www.collegeboard.com. -

Cell Wall Loosening by Expansins1
Plant Physiol. (1998) 118: 333–339 Update on Cell Growth Cell Wall Loosening by Expansins1 Daniel J. Cosgrove* Department of Biology, 208 Mueller Laboratory, Pennsylvania State University, University Park, Pennsylvania 16802 In his 1881 book, The Power of Movement in Plants, Darwin alter the bonding relationships of the wall polymers. The described a now classic experiment in which he directed a growing wall is a composite polymeric structure: a thin tiny shaft of sunlight onto the tip of a grass seedling. The weave of tough cellulose microfibrils coated with hetero- region below the coleoptile tip subsequently curved to- glycans (hemicelluloses such as xyloglucan) and embedded ward the light, leading to the notion of a transmissible in a dense, hydrated matrix of various neutral and acidic growth stimulus emanating from the tip. Two generations polysaccharides and structural proteins (Bacic et al., 1988; later, follow-up work by the Dutch plant physiologist Fritz Carpita and Gibeaut, 1993). Like other polymer compos- Went and others led to the discovery of auxin. In the next ites, the plant cell wall has rheological (flow) properties decade, another Dutchman, A.J.N. Heyn, found that grow- intermediate between those of an elastic solid and a viscous ing cells responded to auxin by making their cell walls liquid. These properties have been described using many more “plastic,” that is, more extensible. This auxin effect different terms: plasticity, viscoelasticity, yield properties, was partly explained in the early 1970s by the discovery of and extensibility are among the most common. It may be “acid growth”: Plant cells grow faster and their walls be- attractive to think that wall stress relaxation and expansion come more extensible at acidic pH. -

Comparison of Seed and Ovule Development in Representative Taxa of the Tribe Cercideae (Caesalpinioideae, Leguminosae) Seanna Reilly Rugenstein Iowa State University
Iowa State University Capstones, Theses and Retrospective Theses and Dissertations Dissertations 1983 Comparison of seed and ovule development in representative taxa of the tribe Cercideae (Caesalpinioideae, Leguminosae) Seanna Reilly Rugenstein Iowa State University Follow this and additional works at: https://lib.dr.iastate.edu/rtd Part of the Botany Commons Recommended Citation Rugenstein, Seanna Reilly, "Comparison of seed and ovule development in representative taxa of the tribe Cercideae (Caesalpinioideae, Leguminosae) " (1983). Retrospective Theses and Dissertations. 8435. https://lib.dr.iastate.edu/rtd/8435 This Dissertation is brought to you for free and open access by the Iowa State University Capstones, Theses and Dissertations at Iowa State University Digital Repository. It has been accepted for inclusion in Retrospective Theses and Dissertations by an authorized administrator of Iowa State University Digital Repository. For more information, please contact [email protected]. INFORMATION TO USERS This reproduction was made from a copy of a document sent to us for microfilming. While the most advanced technology has been used to photograph and reproduce this document, the quality of the reproduction is heavily dependent upon the quality of the material submitted. The following explanation of techniques is provided to help clarify markings or notations which may appear on this reproduction. 1. The sign or "target" for pages apparently lacking from the document photographed is "Missing Page(s)". If it was possible to obtain the missing page(s) or section, they are spliced into the film along with adjacent pages. This may have necessitated cutting through an image and duplicating adjacent pages to assure complete continuity. 2. -

Ovule, Embryo Sac, Embryo and Endosperm Development in Leafy Spurge (Euphorbia Esula L.)1
Reprinted with permission from: Canadian Journal of Botany. 1999. 77:599-610. Published and copyrighted by: National Research Council of Canada, http://www.nrc.ca/cisti/journals/ Ovule, embryo sac, embryo and endosperm development in leafy spurge (Euphorbia 1 esula L.) JEFFREY S. CARMICHAEL* and SARENA M. SELBO Department of Biology, University of North Dakota, Grand Forks, ND 58202, Phone: (701) 777-4666, Fax: (701) 777- 2623, *Author for correspondence, e-mail: [email protected]. Abstract: Leafy spurge (Euphorbia esula) is a noxious, invasive weed that domi- nates many agriculturally important regions. While many research efforts are currently aimed at controlling the spread of this plant, relatively little is known about its sexual reproductive biology, especially from a struc- tural perspective. This report describes key features of ovule development, embryogenesis, and endosperm formation in leafy spurge. Ovules are ana- tropous, bitegmic, and form a zigzag micropyle. A distinct elaisome (ca- runcle) and hypostase are formed as ovules mature. Obturators are present and are derived from placental tissue. The embryo sac conforms to the Po- lygonum type. A single embryo is formed in each seed and stores nutrients primarily as globoid protein bodies. Endosperm is persistent and also con- tains protein bodies as its primary nutrient reserve. Preliminary structural evidence is presented that indicates the potential for apomixis. Keywords: Leafy spurge, Euphorbiaceae, Euphorbia, ovule, endosperm, embryo. Introduction Leafy spurge (Euphorbia esula L.) is an herbaceous perennial that has flourished as a noxious weed of economic and ecological significance (Lajeunesse et al. 1995; Lym and Messersmith 1983; Messersmith 1983; Messersmith and Lym 1983a, b). -

AS Flower Reproduction
2/11/19 AMOEBA SISTERS: VIDEO RECAP ANGIOSPERM REPRODUCTION Amoeba Sisters Video Recap of Plant Reproduction in Angiosperms 1. What characteristics are common in angiosperms? 2. A topic emphasized in this clip is that not all fruits are sweet. Or even edible! Every plant that forms a flower must have a fruit. How would you define a “fruit?” How can fruits be • Flowering plants helpful in seed dispersal? • Bear fruit Fruit is something that has flesh AMOEBA SISTERS: VIDEO RECAP around ANGIOSPERMseeds. REPRODUCTION Amoeba Sisters Video Recap of Plant ReproductionWhen animals in Angiosperms eat them, seeds move away from parent plant. 1. What characteristics are common in angiosperms? 2. A topic emphasized in this clip is that not all fruits are sweet. Or even edible! Every plant that forms a flower must have a fruit. How would you define a “fruit?” How can fruits be helpful in seed dispersal? 3. Flowers can contain one or both genders of flower parts. 4. Flowers can contain one or both genders of flower parts. Label A, B, and C. Label D, E, F, and G. A is the ____________________________________________. D is the ____________________________________________. B is the ____________________________________________. E is the ____________________________________________. C is the ____________________________________________. F is the ____________________________________________. All of these3. Flowers are can contain one or ?both _________________________ genders of flower parts. 4. FlowersG is cathen co ____________________________________________.ntain -

In Vitro Studies on Germination of Immature Ovules and Plant Regeneration from Cotyledons of Impatiens Platypetala Lindl Kyungchul Han Iowa State University
Iowa State University Capstones, Theses and Retrospective Theses and Dissertations Dissertations 1991 In vitro studies on germination of immature ovules and plant regeneration from cotyledons of Impatiens platypetala Lindl Kyungchul Han Iowa State University Follow this and additional works at: https://lib.dr.iastate.edu/rtd Part of the Botany Commons Recommended Citation Han, Kyungchul, "In vitro studies on germination of immature ovules and plant regeneration from cotyledons of Impatiens platypetala Lindl " (1991). Retrospective Theses and Dissertations. 9528. https://lib.dr.iastate.edu/rtd/9528 This Dissertation is brought to you for free and open access by the Iowa State University Capstones, Theses and Dissertations at Iowa State University Digital Repository. It has been accepted for inclusion in Retrospective Theses and Dissertations by an authorized administrator of Iowa State University Digital Repository. For more information, please contact [email protected]. INFORMATION TO USERS This manuscript has been reproduced from the microfilm master. UMI films the text directly from the original or copy submitted. Thus, some thesis and dissertation copies are in typewriter face, while others may be from any type of computer printer. The quality of this reproduction is dependent upon the quality of the copy submitted. Broken or indistinct print, colored or poor quality illustrations and photographs, print bleedthrough, substandard margins, and improper alignment can adversely affect reproduction. In the unlikely event that the author did not send UMI a complete manuscript and there are missing pages, these will be noted. Also, if unauthorized copyright material had to be removed, a note will indicate the deletion. Oversize materials (e.g., maps, drawings, charts) are reproduced by sectioning the original, beginning at the upper left-hand corner and continuing from left to right in equal sections with small overlaps. -

Stamen Petal Filament Anther Carpel Stigma Ovary Style Ovule Sepal
© 2014 Pearson Education, Inc. 1 Stigma Stamen Anther Carpel Style Filament Ovary Petal Sepal Ovule © 2014 Pearson Education, Inc. 2 Sepals Petals Stamens A Carpels B C C gene activity B + C (a) A schematic diagram A B Carpel + gene of the ABC hypothesis gene activity activity Petal A gene Stamen activity Sepal Active B B B B B B B B A A A A genes: A A C C C C A A C C C C C C C C A A C C C C A A A B B A A B B A Whorls: Carpel Stamen Petal Sepal Wild type Mutant lacking A Mutant lacking B Mutant lacking C (b) Side view of flowers with organ identity mutations © 2014 Pearson Education, Inc. 3 Carpel Anther Microsporangium Microsporocytes (2n) Mature flower on sporophyte plant MEIOSIS Microspore (2n) (n) Ovule with Generative cell megasporangium (2n) Tube cell Male gametophyte (in pollen Pollen Germinating Ovary grain) (n) seed MEIOSIS grains Stigma Pollen tube Megasporangium (2n) Sperm Embryo (2n) Surviving Tube nucleus Endosperm (3n) Seed megaspore Seed coat (2n) (n) Antipodal cells Integuments Style Female Polar nuclei gametophyte in central cell (embryo sac) Pollen Synergids tube Zygote (2n) Egg (n) Sperm Nucleus of Egg (n) developing nucleus (n) endosperm (3n) FERTILIZATION Key Haploid (n) Diploid (2n) Discharged sperm nuclei (n) © 2014 Pearson Education, Inc. 4 Abiotic pollination by wind Pollination by insects Common dandelion Common dandelion under normal light under ultraviolet Hazel staminate light flower (stamens only) Hazel carpellate flower (carpels only) © 2014 Pearson Education, Inc. 5 Pollination by bats or birds Long-nosed bat feeding on cactus flower at night Hummingbird drinking nectar of columbine flower © 2014 Pearson Education, Inc. -
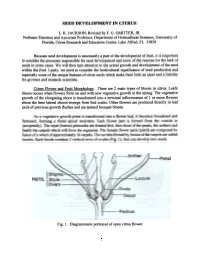
Seed Development in Citrus
SEED DEVELOPMENT IN CITRUS L. K. JACKSON; Revisedby F. G. GMITTER, JR. ProfessorEmeritus and AssociateProfessor, Department of Horticultural Sciences,University of Florida, Citrus Researchand EducationCenter, Lake Alfred, FL 33850 Becauseseed development is necessarilya part of the developmentof fruit. it is important to considerthe processesresponsible for seeddevelopment and someof the reasonsfor the lack of seedsin somecases. We will then turn attentionto the actual growth and developmentof the seed within the fruit. Lastly, we needto considerthe horticultural significanceof seedproduction and especiallysome of the uniquefeatures of citrus seedswhich makethem both an assetand a liability for growersand researchscientists. Citrus Flower and Fruit MoQ1holo~. There are 2 main types of bloom in citrus. Leafy bloom occurswhen flowers form on and with new vegetativegrowth in the spring. The vegetative growth of the elongating shoot is transformedinto a tenninal inflorescenceof 1 or more flowers about the time lateral shootsemerge from bud scales.Other flowers are produceddirectly in leaf axils of previous growth flushesand are termedbouquet bloom. As a vegetative growth point is transfonned into a flower bud, it becomes broadened and flattened, forming a floral apical meristem. Each flower part is fonned from the outside in (acropetally). The sepal (button) primordia are fonned first, then those of the petals, the anthers and finally the carpels which will form the segments. The female flower parts (pistil) are composed by fusion of a whorl of approximately 10 carpels. The cavities fonned by fusion of the carpels are called locules. Each locule contains 2 vertical rows of ovules (Fig. 1), that can develop into seeds. Fig. 1. Diagrammaticportrayal of opencitrus flower. -

Chapter 12: Life Cycles: Meiosis and the Alternation of Generations
Chapter 12 Life Cycles: Meiosis and the Alternation of Generations LIFE CYCLES TRANSFER GENETIC INFORMATION Asexual Reproduction Transfers Unchanged Genetic Information through Mitosis Sexual Reproduction Produces New Information through Meiosis and Fertilization ALTERNATION BETWEEN DIPLOID AND HAPLOID GENERATIONS Plants Vary in the Details of Their Life Cycles Sexual Cycles Can Be Heterosporic or Homosporic Only One Generation Is Multicellular in Zygotic or Gametic Life Cycles The Diploid Generation Has Become Dominant over Evolutionary Time SUMMARY 1 KEY CONCEPTS 1. Life perpetuates itself through reproduction, which is the transfer of genetic information from one generation to the next. This transfer is our definition of life cycle. Reproduction can be asexual or sexual. 2. Asexual reproduction requires a cell division know as mitosis. Asexual reproduction offers many advantages over sexual reproduction, one of which is that it requires only a single parent. A significant disadvantage of asexual reproduction is the loss of genetic diversity and the likelihood of extinction when the environment changes. 3. Sexual reproduction involves the union of two cells, called gametes, which are usually produced by two different individuals. Another kind of cell division, known as meiosis, ultimately is necessary to produce gametes. 4. Every species in the kingdom Plantae has both diploid and haploid phases--that is, plants whose cells are all diploid or all haploid. These phases are called generations, and they alternate with each other over time. 5. The fossil record reveals that the most recent groups to evolve have sporic life cycles, in which the gametophyte (haploid) generation is relatively small and the sporophyte (diploid) generation is dominant in terms of size, complexity, and longevity. -

Reproductive Morphology
Week 3; Wednesday Announcements: 1st lab quiz TODAY Reproductive Morphology Reproductive morphology - any portion of a plant that is involved with or a direct product of sexual reproduction Example: cones, flowers, fruits, seeds, etc. Basic Plant Life cycle Our view of the importance of gametes in the life cycle is shaped by the animal life cycle in which meiosis (the cell division creating haploid daughter cells with only one set of chromosomes) gives rise directly to sperm and eggs which are one celled and do not live independently. Fertilization (or the fusion of gametes – sperm and egg) occurs inside the animal to recreate the diploid organism (2 sets of chromosomes). Therefore, this life cycle is dominated by the diploid generation. This is NOT necessarily the case among plants! Generalized life cycle -overhead- - alternation of generations – In plants, spores are the result of meiosis. These may grow into a multicellular, independent organism (gametophyte – “gamete-bearer”), which eventually produces sperm and eggs (gametes). These fuse (fertilization) and a zygote is formed which grows into what is known as a sporophyte - “spore-bearer”. (In seed plants, pollination must occur before fertilization! ) This sporophyte produces structures called sporangia in which meiosis occurs and the spores are released. Spores (the product of meiosis) are the first cell of the gametophyte generation. Distinguish Pollination from Fertilization and Spore from Gamete Pollination – the act of transferring pollen from anther or male cone to stigma or female cone; restricted to seed plants. Fertilization – the act of fusion between sperm and egg – must follow pollination in seed plants; fertilization occurs in all sexually reproducing organisms. -

The Evolutionary Origin of the Integument in Seed Plants
The evolutionary origin of the integument in seed plants Anatomical and functional constraints as stepping stones towards a new understanding DISSERTATION to obtain the degree Doctor Philosophiae (Doctor of Philosophy, PhD) at the Faculty of Biology and Biotechnology RUHR-UNIVERSITÄT BOCHUM International Graduate School of Biosciences Ruhr-Universität Bochum Evolution and Biodiversity of Plants submitted by Xin Zhang from Urad Qianqi (Inner Mongolia, China) Bochum October 2013 First supervisor: Prof. Dr. Thomas Stützel Second supervisor: Prof. Dr. Ralph Tollrian Der evolutionäre Ursprung des Integuments bei den Samenpflanzen Anatomische und funktionale Untersuchungen als Meilensteine für neue Erkenntnisse DISSERTATION zur Erlangung des Grades eines Doktors der Naturwissenschaften an der Fakultät für Biologie und Biotechnologie RUHR-UNIVERSITÄT BOCHUM Internationale Graduiertenschule Biowissenschaften Ruhr-Universität Bochum angefertigt am Lehrstuhl für Evolution und Biodiversität der Pflanzen vorgelegt von Xin Zhang aus Urad Qianqi (Innere Mongolei, China) Bochum Oktober 2013 Referent: Prof. Dr. Thomas Stützel Korreferent: Prof. Dr. Ralph Tollrian Contents I 1 Introduction 1 1.1 The ovule of gymnosperms and angiosperms 1 1.2 The theories about the origin of the integument in the nineteenth century 1 1.3 The theories about the origin of the integument in the twentieth century 1 1.4 The pollination drop 5 1.5 Ovule and pollination in Cycads 6 1.6 Ovule development in Magnolia stellata (Magnoliaceae) 6 1.7 Aril development in Celastraceae 7 1.8 Seed wing in Catha edulis (Vahl) Endl. (Celastraceae) 8 1.9 Ovule development in Homalanthus populifolius Graham (Euphorbiaceae) and differentiation of caruncula and aril 10 2 Material and methods 12 2.1 Material collection and preparation 12 2.2 Scanning Electron Microscopy (SEM) 12 2.3 Anatomical studies 13 3 Results 14 3.1 The morphology of Zamiaceae 14 3.2 Ovule development and seed anatomy in Zamia L. -

Parts of a Flower.Pub
Angiosperms FLOWERING Plants Complete flowers have stamens, a pistil, petals, and sepals. Stigma (receives the pollen during fertilization) Anther (contains pollen, the male reproductive cell) Pistil Ovary (female reproductive Filament Stamen Stamen organ) (holds the anther) Ovule (reproductive cell which will become the seed when fertilized by pollen) Stamens: Male Reproductive Organs Pistils: Female Reproductive Organs A stamen consists of an anther (which produces The pistil includes an ovary (where the ovules are pollen, the male reproductive cell) and a filament. produced; ovules are the female reproductive cells, the eggs), and a stigma (which receives the pollen during fertilization). Fertilization Pollination is often aided by insects like bees, which fly from flower to flower; as they visit flowers, they spread pollen and deposit it on the stigmas. After pollen grains have landed on the stigma, pollen tubes develop, and burrow down into the ovary, there the pollen (sperm cell) fertilizes an ovule (egg cell). After fertilization, the ovule develops into a seed. In contrast to the idealized diagram above flowers actually are quite varied in appearance. Petals come in a wide variety of shapes and sizes, some “petals” are actually leaves. (Some types of flowers have both male and female reproductive organs (as shown above), others have only male or only female reproductive organs.) Poinsettia Chrysanthemum Lily Primrose female The colorful, showy The blossom is really This lily has both male bracts are actually a cluster of small and female parts. It is modified leaves. The flowers. The showy comparable to the true flowers are outer flowers, which idealized flower yellow and held in the look like petals, are diagram above.