Blood Perfusion and Early Wound Healing Following Implant Placement
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Alveolar Ridge Preservation at Different Anatomical Locations
ALVEOLAR RIDGE PRESERVATION AT DIFFERENT ANATOMICAL LOCATIONS- CLINICAL AND HISTOLOGICAL EVALUATION OF TREATMENT OUTCOME MASTERS THESIS Presented in Partial Fulfillment of Requirements for the Degree Master of Science in Dentistry in the Graduate School of The Ohio State University By Mabel Salas, DDS Graduate Program in Dentistry The Ohio State University 2009 Master’s Examination Committee: Binnaz Leblebicioglu, DDS, MS, PhD, Advisor Dimitris N. Tatakis, DDS, PhD Suda Agarwal, PhD Do-Gyoon Kim, PhD Copyright by Mabel Salas 2009 ABSTRACT Background: Alveolar ridge preservation (ARP) is a surgical technique designed to prevent naturally occurring post-extraction bone resorption. It is well documented that alveolar bone height and width are reduced following tooth extraction as a result of physiologic bone remodeling. Depending on the type of post-extraction intrabony defect, an immediate or early implant placement itself may preserve the bone height and width. However, if the defect is generally too wide for immediate and/or early implant placement, it is recommended to perform ARP surgery to preserve the bone volume for future implant placement. The purpose of this study was to investigate clinical and histological healing outcomes following ARP performed on molar and premolar sites by using freeze-dried bone allograft (FDBA) together with a collagen membrane. Maxillary and mandibular sextants were compared for clinical and histological parameters. Methods: Patients who were scheduled to have tooth extraction and implant placement for a molar or premolar tooth were included into this study. Inclusion criteria were single tooth extraction with intact mesial and distal adjacent teeth. Exclusion criteria were smokers, systemic health problems that may affect wound healing and acute infection ii that may prevent bone graft placement. -

Lecture 2 – Bone
Oral Histology Summary Notes Enoch Ng Lecture 2 – Bone - Protection of brain, lungs, other internal organs - Structural support for heart, lungs, and marrow - Attachment sites for muscles - Mineral reservoir for calcium (99% of body’s) and phosphorous (85% of body’s) - Trap for dangerous minerals (ex:// lead) - Transduction of sound - Endocrine organ (osteocalcin regulates insulin signaling, glucose metabolism, and fat mass) Structure - Compact/Cortical o Diaphysis of long bone, “envelope” of cuboid bones (vertebrae) o 10% porosity, 70-80% calcified (4x mass of trabecular bone) o Protective, subject to bending/torsion/compressive forces o Has Haversian system structure - Trabecular/Cancellous o Metaphysis and epiphysis of long bone, cuboid bone o 3D branching lattice formed along areas of mechanical stress o 50-90% porosity, 15-25% calcified (1/4 mass of compact bone) o High surface area high cellular activity (has marrow) o Metabolic turnover 8x greater than cortical bone o Subject to compressive forces o Trabeculae lined with endosteum (contains osteoprogenitors, osteoblasts, osteoclasts) - Woven Bone o Immature/primitive, rapidly growing . Normally – embryos, newborns, fracture calluses, metaphyseal region of bone . Abnormally – tumors, osteogenesis imperfecta, Pagetic bone o Disorganized, no uniform orientation of collagen fibers, coarse fibers, cells randomly arranged, varying mineral content, isotropic mechanical behavior (behavior the same no matter direction of applied force) - Lamellar Bone o Mature bone, remodeling of woven -

Periodontal Ligament, Cementum, and Alveolar Bone in the Oldest Herbivorous Tetrapods, and Their Evolutionary Significance
Periodontal Ligament, Cementum, and Alveolar Bone in the Oldest Herbivorous Tetrapods, and Their Evolutionary Significance Aaron R. H. LeBlanc*, Robert R. Reisz Department of Biology, University of Toronto Mississauga, Mississauga, Ontario, Canada Abstract Tooth implantation provides important phylogenetic and functional information about the dentitions of amniotes. Traditionally, only mammals and crocodilians have been considered truly thecodont, because their tooth roots are coated in layers of cementum for anchorage of the periodontal ligament, which is in turn attached to the bone lining the alveolus, the alveolar bone. The histological properties and developmental origins of these three periodontal tissues have been studied extensively in mammals and crocodilians, but the identities of the periodontal tissues in other amniotes remain poorly studied. Early work on dental histology of basal amniotes concluded that most possess a simplified tooth attachment in which the tooth root is ankylosed to a pedestal composed of ‘‘bone of attachment’’, which is in turn fused to the jaw. More recent studies have concluded that stereotypically thecodont tissues are also present in non-mammalian, non-crocodilian amniotes, but these studies were limited to crown groups or secondarily aquatic reptiles. As the sister group to Amniota, and the first tetrapods to exhibit dental occlusion, diadectids are the ideal candidates for studies of dental evolution among terrestrial vertebrates because they can be used to test hypotheses of development and homology in deep time. Our study of Permo-Carboniferous diadectid tetrapod teeth and dental tissues reveal the presence of two types of cementum, periodontal ligament, and alveolar bone, and therefore the earliest record of true thecodonty in a tetrapod. -

The Preservation of Alveolar Bone Ridge During Tooth Extraction Marius Kubilius, Ricardas Kubilius, Alvydas Gleiznys
REVIEWS SCIENTIFIC ARTICLES Stomatologija, Baltic Dental and Maxillofacial Journal, 14: 3-11, 2012 The preservation of alveolar bone ridge during tooth extraction Marius Kubilius, Ricardas Kubilius, Alvydas Gleiznys SUMMARY Objectives. The aims were to overview healing of extraction socket, recommendations for atraumatic tooth extraction, possibilities of post extraction socket bone and soft tissues preservation, augmentation. Materials and Methods. A search was done in Pubmed on key words in English from 1962 to December 2011. Additionally, last decades different scientifi c publications, books from ref- erence list were assessed for appropriate review if relevant. Results and conclusions. There was made intraalveolar and extraalveolar postextractional socket healing overview. There was established the importance and effectiveness of atraumatic tooth extraction and subsequent postextractional socket augmentation in limited hard and soft tissue defects. There are many different methods, techniques, periods, materials in regard to the review. It is diffi cult to compare the data and to give the priority to one. Key words: tooth extraction, grafting, socket, healing, ridge preservation. INTRODUCTION Nowadays tooth extraction becomes more im- portunity to get acknowledge with summarized con- portant in complex odontological treatment. Three temporary scientifi c publication results, methodologies dimensional bones’ and soft tissue parameters infl u- and practical recommendations in preserving alveolar ence further treatment plan, results and long time crest in tooth extraction (validity for atraumatic tooth prognosis. Tooth extraction inevitably has infl uence extraction, operative methods, protection of alveolus in bone resorption and changes in gingival contours. after extractions, feasible post extraction fi llers and Further treatment may become more complex in using complications, alternative treatment). -
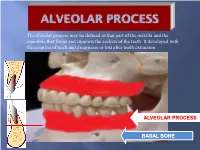
Alveolar Process May Be Defined As That Part of the Maxilla and the Mandible That Forms and Supports the Sockets of the Teeth
The alveolar process may be defined as that part of the maxilla and the mandible that forms and supports the sockets of the teeth. It developed with the eruption of teeth and disappears or lost after tooth extraction ALVEOLAR PROCESS BASAL BONE Alveolar (bone) process: is that part of the maxilla and the mandible that forms and supports the sockets of the teeth. Basal Bone. it is the bone of the facial skeleton which support the alveolar bone. There is no anatomical boundary between basal bones and alveolar bone. Both alveolar process and basal bone are covered by the same periosteum. In some areas alveolar processes may fuse or masked with jaw bones as in (1) Anterior part of maxilla (palatal). (2) Oblique line of the mandible. * Alveolar process is resorbed after extraction of teeth. Functions of alveolar bone – Houses and protects developing permanent teeth, while supporting primary teeth. – Organizes eruption of primary and permanent teeth. – Anchors the roots of teeth to the alveoli, which is achieved by the insertion of Sharpey’s fibers into the alveolar bone proper (attachment). – Helps to move the teeth for better occlusion (support). – Helps to absorb and distribute occlusal forces generated during tooth contact (shock absorber). – Supplies vessels to periodontal ligament. •DEVELOPMENT OF ALVEOLAR BONE •Near the end of the second month of fetal life, the maxilla as well as the mandible form a groove that is open towards the surface of the oral cavity. •Tooth germs develop within the bony structures at late bell stage. •Bony septa and bony bridge begin to form and separatethe individual tooth germs from one another, keeping individual tooth germs in clearly outlined bony compartments. -

Splinting and Occlusal Correction Questions and Answers
6.6 Splinting and Occlusal Correction (Therapy 19 Questions) 11. All of the following may be radiographic signs of trauma from occlusion EXCEPT 1. Widening of the periodontal ligament space 2. Thickening of the lamina dura 3. Root resorption 4. Reduced trabeculation of bone* 35. All of the following are associated with bruxism EXCEPT 1. Sore muscles 2. TMD disturbances 3. Decreased tooth mobility* 4. Occlusal wear 37. Extracoronal splints use restorative materials to stabilize teeth by attaching them to adjacent teeth via removal of tooth structure; intracoronal splints use restorative materials to stabilize teeth by attaching them to adjacent teeth without removal tooth structure. 2. Both statements are FALSE* 60. Which of the following refers to excessive force applied to a tooth or teeth with reduced bone support? 1. Primary occlusal trauma 2. Secondary occlusal trauma* 3. Tertiary occlusal trauma 4. Quaternary occlusal trauma 69. Selective occlusal adjustment is contraindicated in all of the following EXCEPT 1. Elimination of occlusal prematurities* 2. When pulp chambers are large 3. Major occlusal discrepancies that require orthodontics or reconstruction 4. In the presence of sensitivity 82. All of the following are diagnostic of occlusal trauma EXCEPT 1. Wear facets 2. Fremitus 3. Increase in tooth mobility 4. Periodontal pocket formation* 5. Increased width of the periodontal ligament space 158. Unilateral mastication will tend to result in 1. greater accumulation of plaque on the unused side.* 2. greater accumulation of plaque on the used side. 3. a greater degree of periodontal disease on the used side. 4. heavier and moredense bone support on the unused side. -

Palatal Swelling: a Diagnostic Enigma
Hindawi Publishing Corporation Case Reports in Dentistry Volume 2016, Article ID 1945907, 5 pages http://dx.doi.org/10.1155/2016/1945907 Case Report Palatal Swelling: A Diagnostic Enigma Ramalingam Suganya,1 Narasimhan Malathi,1 Harikrishnan Thamizhchelvan,1 Subramaniam Ramkumar,2 andG.V.V.Giri2 1 Department of Oral Pathology and Microbiology, Faculty of Dental Sciences, Sri Ramachandra University, Tamil Nadu, India 2Department of Oral and Maxillofacial Surgery, Faculty of Dental Sciences, Sri Ramachandra University, Tamil Nadu, India Correspondence should be addressed to Ramalingam Suganya; [email protected] Received 24 September 2016; Accepted 25 October 2016 Academic Editor: Luis M. J. Gutierrez Copyright © 2016 Ramalingam Suganya et al. This is an open access article distributed under the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited. Giant cell tumor (GCT) of bone is a giant-cell-rich bony lesion associated with abundant multinucleated osteoclast-type giant cells. It is a primary neoplasm of bone with characteristic clinical, radiological, and pathological features. It is an expansive and lytic lesion without periosteal reaction and prominent peripheral sclerosis. Giant cells are also seen in other diseases like giant cell granuloma of the jaws, traumatic bone cyst, aneurysmal bone cyst, and jaw tumor of hyperparathyroidism. We present a unique case of GCT of palate in a 30-year-old female. 1. Introduction intraoral examination, a massive, solitary proliferative growth measuring 2.5 cm × 3 cm with irregular margins, extending Giant cell tumor of bone or Osteoclastoma is a benign giant from the left maxillary canine region up to the posterior part cell tumor characterized by mononuclear cells proliferation of the hard palate, was evident. -

Oral Histology Lec.1 Lab.1 Preparation of Histological Specimens
Oral Histology Lec.1 Lab.1 Dr.Munir Nasr Preparation of histological specimens Histology (compound of the Greek words: histo “tissue”, and logy “science”) is the study of the microscopic anatomy of cells and tissues of plants and animals. It is commonly performed by examining cells and tissues by sectioning and staining, followed by examination under a light or electron microscopes. Histological studies may be conducted via tissue culture, where live cells can be isolated and maintained in a proper environment outside the body for various research projects. The ability to visualize or differentially identify microscopic structures is frequently enhanced through the use of histological stains. The steps of sample preparations: 1. Tissue fixation 2.Tissue processing 3. Tissue cutting or sectioning 4. Tissue staining Tissue fixation Fixation is a complex series of chemical events that differ for the different groups of substance found in tissues. The aim of fixation: 1- To prevent autolysis and bacterial attack. 2- To fix the tissues so they will not change their volume and shape during processing. 3 - To prepare tissue and leave it in a condition which allow clear staining of sections. 1 4 . To leave tissue as close as their living state as possible, and no small molecules should be lost. Fixation is coming by reaction between the fixative and protein which form a gel, so keeping everything as their in vivo relation to each other. Factors affect fixation: -PH. -Temperature. -Penetration of fixative. -Volume of tissue. According to previous factors we can determine the concentration of fixative and fixation time. Types of fixative: Acetic acid, Formaldehyde, Ethanol, Glutaraldehyde, Methanol and Picric acid. -

Buccal and Lingual Differences of Peri-Implant Bone Quality THESIS
Buccal and Lingual Differences of Peri-Implant Bone Quality THESIS Presented in Partial Fulfillment of the Requirements for the Degree Master of Science in the Graduate School of The Ohio State University By Kathy L. Elias Graduate Program in Dentistry The Ohio State University 2015 Master's Examination Committee: Do-Goon Kim, Advisor William A. Brantley Damian J. Lee Copyright by Kathy L. Elias 2015 Abstract Objective: The objective of the current study was to examine whether peri-implant bone tissue properties at the buccal region are different from those at the lingual region as a result of growth factor treatments at post-implantation healing periods. Methods: Four dental implant groups were used: titanium (Ti) implants, alumina-blasted zirconia implants (ATZ-N), alumina-blasted zirconia implants with demineralized bone matrix (DBM) (ATZ-D), and alumina-blasted zirconia implants with rhBMP-2 (ATZ-B). These implants were placed in mandibles of six male dogs. Nanoindentation elastic modulus (E) and plastic hardness (H) were measured for the buccal and lingual bone tissues adjacent and away from the implants at 3 and 6 weeks post-implantation. A total of 2281 indentations were conducted for 48 placed implants. Results: The peri-implant buccal region had less bone quantity resulting from lower height and narrower width of bone tissue than the lingual region. Buccal bone tissues had significant greater mean values of E and H than lingual bone tissues at each distance and healing period (p<0.007). Nearly all implant treatment groups displayed lower mean values of the E at the lingual bone tissues than at the buccal bone tissues (p<0.046) although the difference was not significant for the Ti implant group (p=0.758). -

I. Healing of the Extraction Site
I. Healing of the Extraction Site Bach Le, DDS, MD, FICD, FACD F. Kyle Yip, MS, DDS, MD Normal extraction physiology Early histologic studies in the mid-20th century of human and animal extraction sockets by Mangos,1 Christopher, 2 Amler, 3, 4 and Boyne, 5 explored in detail the early and late phases of socket healing. Evian further characterized socket healing between four and 16 weeks in 1982 utilizing biopsies of sockets and core biopsies. 6 The following sequence was generally seen in healthy sockets: 1. Day 1 – Clot formation 2. Day 2-7 – Granulation tissue fills socket 3. Day 4-20 – Connective tissue replaces granulation tissue; spindle cells, collagen fibers, and early vascularity is seen 4. Day 7 – Bone formation begins with uncalcified spicules and osteoid at the socket base and periphery 5. Day 20 – Mineralization begins 6. Day 40 – two-thirds socket filled with immature bone, lamina dura becomes lost 7. Day 50-90 – Bone matures into trabecular pattern resembling alveolus 8. Day 100 – Socket density comparable to surrounding bone, minimal residual osteogenic activity Socket Epithelialization Proliferation of epithelium at the periphery of the socket was noted by Amler to begin at day 4. 3 Amler and Mangos found complete fusion of the overlying epithelium around day 20-30, although some sockets were noted to remain incompletely covered at day 35. 1, 3 Amler noted that epithelialization was delayed by sloughing epithelium at edges of ragged and traumatized native epithelium, but minimal sloughing was found at the edges where clean incisions were made. 3 Dimensional Changes of the Socket and Ridge The alveolar process is comprised of both cortical and bundle bone. -

Minimally Invasive Accelerated Orthodontic Techniques: a Clinical, Radiological, and Histological Comparison on a Rat Model
Nova Southeastern University NSUWorks Student Theses, Dissertations and Capstones College of Dental Medicine 2018 Minimally Invasive Accelerated Orthodontic Techniques: A Clinical, Radiological, and Histological Comparison on a Rat Model Sausha Toghranegar Nova Southeastern University Follow this and additional works at: https://nsuworks.nova.edu/hpd_cdm_stuetd Part of the Dentistry Commons All rights reserved. This publication is intended for use solely by faculty, students, and staff of Nova Southeastern University. No part of this publication may be reproduced, distributed, or transmitted in any form or by any means, now known or later developed, including but not limited to photocopying, recording, or other electronic or mechanical methods, without the prior written permission of the author or the publisher. NSUWorks Citation Sausha Toghranegar. 2018. Minimally Invasive Accelerated Orthodontic Techniques: A Clinical, Radiological, and Histological Comparison on a Rat Model. Master's thesis. Nova Southeastern University. Retrieved from NSUWorks, College of Dental Medicine. (79) https://nsuworks.nova.edu/hpd_cdm_stuetd/79. This Thesis is brought to you by the College of Dental Medicine at NSUWorks. It has been accepted for inclusion in Student Theses, Dissertations and Capstones by an authorized administrator of NSUWorks. For more information, please contact [email protected]. Minimally Invasive Accelerated Orthodontic Techniques: A Clinical, Radiological, and Histological Comparison on a Rat Model Sausha Toghranegar DMD Saynur Vardar-Sengul -

Glossary of Periodontal Terms.Pdf
THE AMERICAN ACADEMY OF PERIODONTOLOGY Glossary of Periodontal Te rms 4th Edition Copyright 200 I by The American Academy of Periodontology Suite 800 737 North Michigan Avenue Chicago, Illinois 60611-2690 All rights reserved. No part of this publication may be reproduced, stored in a retrieval system, or transmitted in any form or by any means, electronic, mechanical, photocopying, or otherwise without the express written permission of the publisher. ISBN 0-9264699-3-9 The first two editions of this publication were published under the title Glossary of Periodontic Terms as supplements to the Journal of Periodontology. First edition, January 1977 (Volume 48); second edition, November 1986 (Volume 57). The third edition was published under the title Glossary vf Periodontal Terms in 1992. ACKNOWLEDGMENTS The fourth edition of the Glossary of Periodontal Terms represents four years of intensive work by many members of the Academy who generously contributed their time and knowledge to its development. This edition incorporates revised definitions of periodontal terms that were introduced at the 1996 World Workshop in Periodontics, as well as at the 1999 International Workshop for a Classification of Periodontal Diseases and Conditions. A review of the classification system from the 1999 Workshop has been included as an Appendix to the Glossary. Particular recognition is given to the members of the Subcommittee to Revise the Glossary of Periodontic Terms (Drs. Robert E. Cohen, Chair; Angelo Mariotti; Michael Rethman; and S. Jerome Zackin) who developed the revised material. Under the direction of Dr. Robert E. Cohen, the Committee on Research, Science and Therapy (Drs. David L.