Minimally Invasive Accelerated Orthodontic Techniques: a Clinical, Radiological, and Histological Comparison on a Rat Model
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Alveolar Ridge Preservation at Different Anatomical Locations
ALVEOLAR RIDGE PRESERVATION AT DIFFERENT ANATOMICAL LOCATIONS- CLINICAL AND HISTOLOGICAL EVALUATION OF TREATMENT OUTCOME MASTERS THESIS Presented in Partial Fulfillment of Requirements for the Degree Master of Science in Dentistry in the Graduate School of The Ohio State University By Mabel Salas, DDS Graduate Program in Dentistry The Ohio State University 2009 Master’s Examination Committee: Binnaz Leblebicioglu, DDS, MS, PhD, Advisor Dimitris N. Tatakis, DDS, PhD Suda Agarwal, PhD Do-Gyoon Kim, PhD Copyright by Mabel Salas 2009 ABSTRACT Background: Alveolar ridge preservation (ARP) is a surgical technique designed to prevent naturally occurring post-extraction bone resorption. It is well documented that alveolar bone height and width are reduced following tooth extraction as a result of physiologic bone remodeling. Depending on the type of post-extraction intrabony defect, an immediate or early implant placement itself may preserve the bone height and width. However, if the defect is generally too wide for immediate and/or early implant placement, it is recommended to perform ARP surgery to preserve the bone volume for future implant placement. The purpose of this study was to investigate clinical and histological healing outcomes following ARP performed on molar and premolar sites by using freeze-dried bone allograft (FDBA) together with a collagen membrane. Maxillary and mandibular sextants were compared for clinical and histological parameters. Methods: Patients who were scheduled to have tooth extraction and implant placement for a molar or premolar tooth were included into this study. Inclusion criteria were single tooth extraction with intact mesial and distal adjacent teeth. Exclusion criteria were smokers, systemic health problems that may affect wound healing and acute infection ii that may prevent bone graft placement. -

Lecture 2 – Bone
Oral Histology Summary Notes Enoch Ng Lecture 2 – Bone - Protection of brain, lungs, other internal organs - Structural support for heart, lungs, and marrow - Attachment sites for muscles - Mineral reservoir for calcium (99% of body’s) and phosphorous (85% of body’s) - Trap for dangerous minerals (ex:// lead) - Transduction of sound - Endocrine organ (osteocalcin regulates insulin signaling, glucose metabolism, and fat mass) Structure - Compact/Cortical o Diaphysis of long bone, “envelope” of cuboid bones (vertebrae) o 10% porosity, 70-80% calcified (4x mass of trabecular bone) o Protective, subject to bending/torsion/compressive forces o Has Haversian system structure - Trabecular/Cancellous o Metaphysis and epiphysis of long bone, cuboid bone o 3D branching lattice formed along areas of mechanical stress o 50-90% porosity, 15-25% calcified (1/4 mass of compact bone) o High surface area high cellular activity (has marrow) o Metabolic turnover 8x greater than cortical bone o Subject to compressive forces o Trabeculae lined with endosteum (contains osteoprogenitors, osteoblasts, osteoclasts) - Woven Bone o Immature/primitive, rapidly growing . Normally – embryos, newborns, fracture calluses, metaphyseal region of bone . Abnormally – tumors, osteogenesis imperfecta, Pagetic bone o Disorganized, no uniform orientation of collagen fibers, coarse fibers, cells randomly arranged, varying mineral content, isotropic mechanical behavior (behavior the same no matter direction of applied force) - Lamellar Bone o Mature bone, remodeling of woven -

Periodontal Ligament, Cementum, and Alveolar Bone in the Oldest Herbivorous Tetrapods, and Their Evolutionary Significance
Periodontal Ligament, Cementum, and Alveolar Bone in the Oldest Herbivorous Tetrapods, and Their Evolutionary Significance Aaron R. H. LeBlanc*, Robert R. Reisz Department of Biology, University of Toronto Mississauga, Mississauga, Ontario, Canada Abstract Tooth implantation provides important phylogenetic and functional information about the dentitions of amniotes. Traditionally, only mammals and crocodilians have been considered truly thecodont, because their tooth roots are coated in layers of cementum for anchorage of the periodontal ligament, which is in turn attached to the bone lining the alveolus, the alveolar bone. The histological properties and developmental origins of these three periodontal tissues have been studied extensively in mammals and crocodilians, but the identities of the periodontal tissues in other amniotes remain poorly studied. Early work on dental histology of basal amniotes concluded that most possess a simplified tooth attachment in which the tooth root is ankylosed to a pedestal composed of ‘‘bone of attachment’’, which is in turn fused to the jaw. More recent studies have concluded that stereotypically thecodont tissues are also present in non-mammalian, non-crocodilian amniotes, but these studies were limited to crown groups or secondarily aquatic reptiles. As the sister group to Amniota, and the first tetrapods to exhibit dental occlusion, diadectids are the ideal candidates for studies of dental evolution among terrestrial vertebrates because they can be used to test hypotheses of development and homology in deep time. Our study of Permo-Carboniferous diadectid tetrapod teeth and dental tissues reveal the presence of two types of cementum, periodontal ligament, and alveolar bone, and therefore the earliest record of true thecodonty in a tetrapod. -

The Preservation of Alveolar Bone Ridge During Tooth Extraction Marius Kubilius, Ricardas Kubilius, Alvydas Gleiznys
REVIEWS SCIENTIFIC ARTICLES Stomatologija, Baltic Dental and Maxillofacial Journal, 14: 3-11, 2012 The preservation of alveolar bone ridge during tooth extraction Marius Kubilius, Ricardas Kubilius, Alvydas Gleiznys SUMMARY Objectives. The aims were to overview healing of extraction socket, recommendations for atraumatic tooth extraction, possibilities of post extraction socket bone and soft tissues preservation, augmentation. Materials and Methods. A search was done in Pubmed on key words in English from 1962 to December 2011. Additionally, last decades different scientifi c publications, books from ref- erence list were assessed for appropriate review if relevant. Results and conclusions. There was made intraalveolar and extraalveolar postextractional socket healing overview. There was established the importance and effectiveness of atraumatic tooth extraction and subsequent postextractional socket augmentation in limited hard and soft tissue defects. There are many different methods, techniques, periods, materials in regard to the review. It is diffi cult to compare the data and to give the priority to one. Key words: tooth extraction, grafting, socket, healing, ridge preservation. INTRODUCTION Nowadays tooth extraction becomes more im- portunity to get acknowledge with summarized con- portant in complex odontological treatment. Three temporary scientifi c publication results, methodologies dimensional bones’ and soft tissue parameters infl u- and practical recommendations in preserving alveolar ence further treatment plan, results and long time crest in tooth extraction (validity for atraumatic tooth prognosis. Tooth extraction inevitably has infl uence extraction, operative methods, protection of alveolus in bone resorption and changes in gingival contours. after extractions, feasible post extraction fi llers and Further treatment may become more complex in using complications, alternative treatment). -

Combination of Bone Allograft, Barrier Membrane and Doxycycline in the Treatment of Infrabony Periodontal Defects: a Comparative Trial
CORE Metadata, citation and similar papers at core.ac.uk Provided by Elsevier - Publisher Connector The Saudi Dental Journal (2015) 27, 155–160 King Saud University The Saudi Dental Journal www.ksu.edu.sa www.sciencedirect.com ORIGINAL ARTICLE Combination of bone allograft, barrier membrane and doxycycline in the treatment of infrabony periodontal defects: A comparative trial Ashish Agarwal a,*, N.D. Gupta b a Department of Periodontics, Institute of Dental Sciences, Bareilly, India b Department of Periodontics, DR. Z.A. Dental College, Aligarh Muslim University, Aligarh, India Received 1 March 2014; revised 4 December 2014; accepted 26 January 2015 Available online 27 May 2015 KEYWORDS Abstract Aim: The purpose of the present study was to compare the regenerative potential of Bone grafting; noncontained periodontal infrabony defects treated with decalcified freeze-dried bone allograft Doxycycline; (DFDBA) and barrier membrane with or without local doxycycline. Guided tissue regeneration Methods: This study included 48 one- or two-wall infrabony defects from 24 patients (age: 30–65 years) seeking treatment for chronic periodontitis. Defects were randomly divided into two groups and were treated with a combination of DFDBA and barrier membrane, either alone (combined treatment group) or with local doxycycline (combined treatment + doxycycline group). At baseline (before surgery) and 3 and 6 months after surgery, the pocket probing depth (PPD), clinical attachment level (CAL), radiological bone fill (RBF), and alveolar height reduction (AHR) were recorded. Analysis of variance and the Newman–Keuls post hoc test were used for sta- tistical analysis. A two-tailed p-value of less than 0.05 was considered to be statistically significant. -

Modifications of Polymeric Membranes Used in Guided Tissue and Bone Regeneration
polymers Review Modifications of Polymeric Membranes Used in Guided Tissue and Bone Regeneration Wojciech Florjanski 1 , Sylwia Orzeszek 1, Anna Olchowy 1, Natalia Grychowska 2, Wlodzimierz Wieckiewicz 2, Andrzej Malysa 1, Joanna Smardz 1 and Mieszko Wieckiewicz 1,* 1 Department of Experimental Dentistry, Faculty of Dentistry, Wroclaw Medical University, 50-367 Wroclaw, Poland; wojtek.fl[email protected] (W.F.); [email protected] (S.O.); [email protected] (A.O.); [email protected] (A.M.); [email protected] (J.S.) 2 Department of Prosthetic Dentistry, Faculty of Dentistry, Wroclaw Medical University, 50-367 Wroclaw, Poland; [email protected] (N.G.); [email protected] (W.W.) * Correspondence: [email protected] Received: 14 March 2019; Accepted: 28 April 2019; Published: 2 May 2019 Abstract: Guided tissue/bone regeneration (GTR/GBR) is a widely used procedure in contemporary dentistry. To achieve the required results of tissue regeneration, soft tissues that reproduce quickly are separated from the slow-growing bone tissue by membranes. Many types of membranes are currently in use, but none of them fulfil all of the desired features. To address this issue, further research on developing new membranes with better separation characteristics, such as membrane modification, is needed. Many of the current innovative modified materials are still in the phase of in vitro and experimental studies. A collective review on new trends in membrane modification to GTR/GBR is needed due to the widespread use of polymeric membranes and the constant development in the field of dentistry. Therefore, the aim of this review was to present an overview of polymeric membrane modifications to the GTR/GBR reported in the literature. -

Iowa Section of the American Association for Dental Research
Iowa Section of the American Association for Dental Research 67th Annual Meeting Moving Oral Health Research Forward Through Collaboration Our Keynote Speaker — Dr. Mary L. Marazita is professor and vice chair of the Department of Oral Biology in the University of Pittsburgh School of Dental Medicine and the Director of the Center for Craniofacial and Den- tal Genetics. With over 400 publications and almost 35 years of continuous NIH-funding, Dr. Marazita is a world leader in the use of statistical genetics and genetic epidemiology for understanding craniofacial birth defects and oral-facial development. In 1980, Dr. Marazita earned a Ph.D. in Genetics from the University of North Carolina, and in 1982, she completed post-doctoral train- ing in craniofacial biology at the University of Southern California. Before coming to Pittsburg, Dr. Marazita had faculty appointments at UCLA and the Medical College of Virginia. She is also a diplo- mate of the American Board of Medical Genetics and a Founding Fellow of the American College of Medical Genetics. At the University of Pittsburgh, Dr. Marazita has held numerous other appointments in the School of Dental Medicine, including assistant dean, associate dean for research, head of the Division of Mary L Marazita, Ph.D. Oral Biology, and chair of the Department of Oral Biology. Given her international reputation and commitment to the oral sci- ences, Dr. Marazita has held important roles in the National Institutes of Health (NIH), including the National Institute of Dental and Craniofacial Research (NIDCR), and the National Human Genome Research Institute (NHGRI). Dr. Marazita exemplifies the collaborative nature of scientific research, and embodies the theme of this conference. -
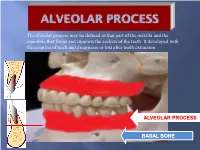
Alveolar Process May Be Defined As That Part of the Maxilla and the Mandible That Forms and Supports the Sockets of the Teeth
The alveolar process may be defined as that part of the maxilla and the mandible that forms and supports the sockets of the teeth. It developed with the eruption of teeth and disappears or lost after tooth extraction ALVEOLAR PROCESS BASAL BONE Alveolar (bone) process: is that part of the maxilla and the mandible that forms and supports the sockets of the teeth. Basal Bone. it is the bone of the facial skeleton which support the alveolar bone. There is no anatomical boundary between basal bones and alveolar bone. Both alveolar process and basal bone are covered by the same periosteum. In some areas alveolar processes may fuse or masked with jaw bones as in (1) Anterior part of maxilla (palatal). (2) Oblique line of the mandible. * Alveolar process is resorbed after extraction of teeth. Functions of alveolar bone – Houses and protects developing permanent teeth, while supporting primary teeth. – Organizes eruption of primary and permanent teeth. – Anchors the roots of teeth to the alveoli, which is achieved by the insertion of Sharpey’s fibers into the alveolar bone proper (attachment). – Helps to move the teeth for better occlusion (support). – Helps to absorb and distribute occlusal forces generated during tooth contact (shock absorber). – Supplies vessels to periodontal ligament. •DEVELOPMENT OF ALVEOLAR BONE •Near the end of the second month of fetal life, the maxilla as well as the mandible form a groove that is open towards the surface of the oral cavity. •Tooth germs develop within the bony structures at late bell stage. •Bony septa and bony bridge begin to form and separatethe individual tooth germs from one another, keeping individual tooth germs in clearly outlined bony compartments. -

Peri-Implantitis Regenerative Therapy: a Review
biology Review Peri-Implantitis Regenerative Therapy: A Review Lorenzo Mordini 1,* , Ningyuan Sun 1, Naiwen Chang 1, John-Paul De Guzman 1, Luigi Generali 2 and Ugo Consolo 2 1 Department of Periodontology, Tufts University School of Dental Medicine, Boston, MA 02111, USA; [email protected] (N.S.); [email protected] (N.C.); [email protected] (J.-P.D.G.) 2 Department of Surgery, Medicine, Dentistry and Morphological Sciences with Transplant Surgery, Oncology and Regenerative Medicine Relevance (CHIMOMO), University of Modena and Reggio Emilia, 41124 Modena, Italy; [email protected] (L.G.); [email protected] (U.C.) * Correspondence: [email protected] Simple Summary: Regenerative therapies are one of the options to treat peri-implantitis diseases that cause peri-implant bone loss. This review reports classic and current literature to describe the available knowledge on regenerative peri-implant techniques. Abstract: The surgical techniques available to clinicians to treat peri-implant diseases can be divided into resective and regenerative. Peri-implant diseases are inflammatory conditions affecting the soft and hard tissues around dental implants. Despite the large number of investigations aimed at identifying the best approach to treat these conditions, there is still no universally recognized protocol to solve these complications successfully and predictably. This review will focus on the regenerative treatment of peri-implant osseous defects in order to provide some evidence that can aid clinicians in the approach to peri-implant disease treatment. Keywords: peri-implant disease; peri-implant mucositis; peri-implantitis; re-osseointegration; regen- Citation: Mordini, L.; Sun, N.; Chang, erative therapy N.; De Guzman, J.-P.; Generali, L.; Consolo, U. -

Treatment of Different Types of Bone Defects with Concentrated Growth Factor
Gökmenoğlu et al. Int J Oral Dent Health 2016, 2:029 International Journal of Volume 2 | Issue 2 ISSN: 2469-5734 Oral and Dental Health Case Series: Open Access Treatment of Different Types of Bone Defects with Concentrated Growth Factor: Four Case Reports Ceren Gökmenoğlu1, Mustafa Cihan Yavuz1, Elif Sadik2, Varol Çanakçi1 and Cankat Kara1* 1Department of Periodontology, Faculty of Dentistry, Ordu University, Ordu, Turkey 2Department of Oral and Maxillofacial Radiology, Faculty of Dentistry, Ordu University, Ordu, Turkey *Corresponding author: Cankat KARA, DDS, PhD, Associate Professor, Department of Periodontology, Faculty of Dentistry, Ordu University, 52100 Ordu, Turkey, Tel: +90 452 212 1283, Email: [email protected] barrier to epithelium migration [4]. The purpose of these case series Abstract is to document the beneficial role of CGF mixed with bone graft and Various techniques have been attempted in the past to truly CGF barrier membrane to accelerate bone formation in the healing of regenerate the lost bone structures. Owing to its stimulatory effect different bone defect areas. on angiogenesis and epithelialization, concentrated growth factor (CGF) is an excellent material for enhancing bone healing. The Cases Report purpose of these case series is to document the beneficial role of CGF in the healing of different bone defect areas. This report This report describes four female patients presented with (lateral describes four female patients presented with (lateral cyst on cyst on tooth 22; periimplantitis on mandibular left first premolar tooth 22; periimplantitis on mandibular left first premolar area; area; furcation lesion on tooth 36, periapical abcess on teeth 11-21) furcation lesion on tooth 36, periapical abcess on teeth 11-21). -

Endodontic Recommended Reading List 2018 A. ความรู้วิทยาศาสตร์พื้นฐาน 1
1 Endodontic Recommended Reading List 2018 A. ความรู้วิทยาศาสตร์พื้นฐาน 1. Gross, microscopic และ ultrastructural anatomy of soft and hard tissue (tooth and surrounding) 2. Embryology, histology, bone biology 3. Microbiology 4. Oral infection & immunology 5. Oral medicine, Oral pathology 6. Inflammation & healing 7. ชีวสถิติ 8. ความรู้กฎหมายวิชาชีพ เจตคติ และจรรยาบรรณแห่งวิชาชีพทันตกรรม B. วิทยาศาสตร์การแพทย์ที่เกี่ยวข้องกับสาขา 1. Embryology, histology, physiology of pulp and periapical tissue 2. Biology of dental pulp 1. Bennett CG, Kelln EE, Biddington WR. Age changes of the vascular pattern of the human dental pulp. Arch Oral Biol. 1965;10(6):995-8. 2. Bernick S. Effect of aging on the nerve supply to human teeth. J Dent Res. 1967;46(4):694- 9. 3. Bernick S. Lymphatic vessels of the human dental pulp. J Dent Res. 1977;56(1):70-7. 4. Brannstrom M. The hydrodynamic theory of dentinal pain: sensation in preparations, caries, and the dentinal crack syndrome. J Endod. 1986;12(10):453-7. 2 5. Brannstrom M, Linden LA, Johnson G. Movement of dentinal and pulpal fluid caused by clinical procedures. J Dent Res. 1968;47(5):679-82. 6. Byers MR, Neuhaus SJ, Gehrig JD. Dental sensory receptor structure in human teeth. Pain. 1982;13(3):221-35. 7. Carrigan PJ, Morse DR, Furst ML, Sinai IH. A scanning electron microscopic evaluation of human dentinal tubules according to age and location. J Endod. 1984;10(8):359-63. 8. DENTISTRY AAOP. Guideline on Pulp Therapy for Primary and Immature Permanent Teeth. Pediatr Dent. 2016;38(6):280-8. 9. Fitzgerald M, Chiego DJ, Jr., Heys DR. Autoradiographic analysis of odontoblast replacement following pulp exposure in primate teeth. -

Bioresorbable Collagen Membranes for Guided Bone Regeneration
6 Bioresorbable Collagen Membranes for Guided Bone Regeneration Haim Tal, Ofer Moses, Avital Kozlovsky and Carlos Nemcovsky Department of Periodontology and Implantology, Tel Aviv University Israel 1. Introduction Localized lack of bone volume in the jaws may be due to congenital, post-traumatic, postsurgical defects or different disease processes. Increasing the bone volume has long been an attractive field of basic and clinical research. The introduction of implant therapy, and the proven relationship between long-term prognosis of dental implants and adequate bone volume at the implant site (Lekholm et al. 1986), dramatically increased the interest of both clinicians and scientists in this field, making augmentation procedures an important part of contemporary implant therapy. Basically, four methods have been described to augment bone volume: a. osteoinduction, using appropriate growth factors (Reddi 1981; Urist 1965); b. osteoconduction, using grafting materials that serve as scaffolds for new bone growth (Buch et al. 1986; Reddi et al. 1987); c. distraction osteogenesis, by which a surgically induced bone fracture enables slow controlled pulling apart of the separated bone fragments (Ilizarov 1989a,b); d. guided bone regeneration, which allows selective bone tissue growth into a space maintained by tissue barriers (Dahlin et al. 1988, 1991a; Kostopoulos & Karring 1994; Nyman & Lang 1994). Among the different methods, guided bone regeneration (GBR) is the most popular and best documented for the treatment of localized bone defects in the jaws, probably due to its relative simplicity of use while allowing the placement of endosseous implants in areas of the jaw with bony defects and/or insufficient bone volume. Highly predictable success rates can be achieved using GBR; in fact, it has been shown that success rates of implants placed at GBR treated sites and sites without bone augmentation are comparable (Hammerle et al.