(12) Patent Application Publication (10) Pub. No.: US 2006/0159818 A1 Kunieda (43) Pub
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Collection of Products Made Through Affrinnovation ‐ 6Th Industrialization of Agriculture,Forestry and Fisheries ‐
Collection of Products made through AFFrinnovation ‐ 6th Industrialization of Agriculture,Forestry and Fisheries ‐ January 2016 Ministry of Agriculture, Forestry and Fisheries In Japan, agricultural, forestry and fisheries workers have been making efforts to raise their income by processing and selling their products in an integrated manner to create added value. These efforts are called the “AFFrinnovation,” and agricultural, forestry and fisheries workers throughout the country have made the best use of inventiveness to produce a variety of products. This book introduces products that were created through the efforts to promote the AFFrinnovation. We hope this book would arouse your interest in the AFFrinnovation in Japan. Notes ○ Information contained in this book is current as of the editing in January 2016, and therefore not necessarily up to date. ○ This book provides information of products by favor of the business operators as their producers. If you desire to contact or visit any of business operators covered in this book, please be careful not to disturb their business activities. [Contact] Food Industrial Innovation Division Food Industry Affairs Bureau Ministry of Agriculture, Forestry and Fisheries URL:https://www.contact.maff.go.jp/maff/form/114e.html Table of Contents Hokkaido Name of Product Name Prefecture Page Business Operator Tomatoberry Juice Okamoto Nouen Co., Ltd. Hokkaido 1 Midi Tomato Juice Okamoto Nouen Co., Ltd. Hokkaido 2 Tokachi Marumaru Nama Cream Puff (fresh cream puff) Okamoto Nouen Co., Ltd. Hokkaido 3 (tomato, corn, and azuki bean flavors) Noka‐no Temae‐miso (Farm‐made fermented soybean Sawada Nojo LLC Hokkaido 4 paste) Asahikawa Arakawa Green Cheese Miruku‐fumi‐no‐ki (milky yellow) Hokkaido 5 Bokujo LLC Asahikawa Arakawa Farm Green Cheese Kokuno‐aka (rich red) Hokkaido 6 LLC Menu at a farm restaurant COWCOW Café Oono Farm Co., Ltd. -

ESORA: the Rise of the Culinary Bambinos | High Net Worth
15/01/2019 ESORA: The Rise of the Culinary Bambinos | High Net Worth Dining (https://www.hnworth.com/article-category/dining) ESORA: The Rise of the Culinary Bambinos By Sihan Lee https://www.hnworth.com/article/2018/12/06/esora-the-rise-of-the-culinary-bambinos/ 1/9 15/01/2019 ESORA: The Rise of the Culinary Bambinos | High Net Worth December 6, 2018 Recommended 2018 has been an epic year of change for the F&B scene in Singapore, Insights (https://www.hnworth.com/article- notwithstanding the sad category/insights) departure/closure of several renowned institutions in the industry. With several big names out of action, this creates room for the younger, more ambitious chefs and restauranteurs to strive and crack the glass ceilings of fine dining. And for many of these up-and-comers, beige is (https://www.hnworth.com/article/201 the new white; and wood is the new 10-biggest-risks-for-2018/) marble. The 10 Biggest Risks for 2018 (https://www.hnworth.com/article/201 10-biggest-risks-for-2018/) Style (https://www.hnworth.com/article- category/style) (http://www.hnworth.com/wp- content/uploads/2018/11/Esora-Art- 1.jpg) Contributing significantly to these efforts, is the newly opened ESORA (https://www.restaurant-esora.com/), (https://www.hnworth.com/article/201 an intimate 26-seater Kappo-style to-get-the-nike-react-element-87- establishment that has recently added x-undercover/) the T.Dining Best New Restaurant award to its impending accolades, just https://www.hnworth.com/article/2018/12/06/esora-the-rise-of-the-culinary-bambinos/ 2/9 15/01/2019 ESORA: The Rise of the Culinary Bambinos | High Net Worth 3 months of opening its doors. -

Food Byways: the Sugar Road by Masami Ishii
Vol. 27 No. 3 October 2013 Kikkoman’s quarterly intercultural forum for the exchange of ideas on food SPOTLIGHT JAPAN: JAPANESE STYLE: Ohagi and Botamochi 5 MORE ABOUT JAPANESE COOKING 6 Japan’s Evolving Train Stations 4 DELECTABLE JOURNEYS: Nagano Oyaki 5 KIKKOMAN TODAY 8 THE JAPANESE TABLE Food Byways: The Sugar Road by Masami Ishii This third installment of our current Feature series traces Japan’s historical trade routes by which various foods were originally conveyed around the country. This time we look at how sugar came to make its way throughout Japan. Food Byways: The Sugar Road From Medicine to Sweets to be imported annually, and it was eventually According to a record of goods brought to Japan from disseminated throughout the towns of Hakata China by the scholar-priest Ganjin (Ch. Jianzhen; and Kokura in what is known today as Fukuoka 688–763), founder of Toshodaiji Temple in Nara, Prefecture in northern Kyushu island. As sugar cane sugar is thought to have been brought here in made its way into various regions, different ways the eighth century. Sugar was considered exceedingly of using it evolved. Reflecting this history, in the precious at that time, and until the thirteenth 1980s the Nagasaki Kaido highway connecting the century it was used solely as an ingredient in the cities of Nagasaki and Kokura was dubbed “the practice of traditional Chinese medicine. Sugar Road.” In Japan, the primary sweeteners had been After Japan adopted its national seclusion laws maltose syrup made from glutinous rice and malt, in the early seventeenth century, cutting off trade and a sweet boiled-down syrup called amazura made and contact with much of the world, Nagasaki from a Japanese ivy root. -
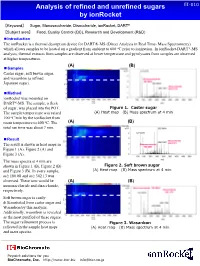
Analysis of Refined and Unrefined Sugars By
Analysis of refined and unrefined sugars EI-010 by ionRocket 【Keyword】 Sugar, Monosaccharide, Disaccharide, ionRocket, DART® 【Subject area】 Food, Quality Control (QC), Research and Development (R&D) ■Introduction The ionRocket is a thermal desorption device for DART®-MS (Direct Analysis in Real Time- Mass Spectrometry) which allows samples to be heated on a gradient from ambient to 600 oC prior to ionization.. In ionRocket-DART®-MS analysis, thermal extracts from samples are observed at lower temperature and pyrolysates from samples are observed at higher temperatures. (A) (B) ■Samples Caster sugar, soft brown sugar, and wasanbon (a refined Japanese sugar). ■Method ionRocket was mounted on DART®-MS. The sample, a fleck of sugar, was placed into the POT. Figure 1. Caster sugar The sample temperature was raised (A) Heat map (B) Mass spectrum at 4 min 100 oC/min by the ionRocket from room temperature to 600 oC. The (A) (B) total run time was about 7 min. ■Result The result is shown in heat maps in Figure 1 (A), Figure 2 (A) and Figure 3 (A). The mass spectra at 4 min are shown in Figure 1 (B), Figure 2 (B) Figure 2. Soft brown sugar and Figure 3 (B). In every sample, (A) Heat map (B) Mass spectrum at 4 min m/z 180.08 and m/z 342.13 was observed. These ions would be (A) (B) monosaccharide and disaccharide, respectively. Soft brown sugar is easily differentiated from caster sugar and Wasanbon by this analysis. Additionally, wasonbon is revealed as the most purified of these sugars. The sugar refinement process is Figure 3. -

Souvenirs Experience
Shopping SOUVENIRS The sun-dried Salt which is made in Naoshima. Red pumpkin postcard ¥100 Naoshima limitation postcard of Kusama Yayoi which keeps attracting attention at the whole Salt donut world. 6 pieces / ¥440 It is a doughnut which the salt with full of minerals was Salt soda pop kneaded into. 200ml / ¥220 Sweetness and saltiness match it moderately and are refreshing taste. Wasanbon-to sugar(refined Japanese sugar) Bag of Salt/ ¥500 (90g) Jar of Salt/ ¥500 (65g) 12pieces/¥660 SOLASHIO makes use of a salt manufacture This is a delicious wasanbon with Kagawa's high-quality technology carried out flourishingly in Naoshima in old sugar wasanbon-to sugar. days and is the perfection sun-dried salt which bathes It is mellow, and the sweetness without the habit fits green tea and coffee, tea well. in sunlight slowly and carefully in Naoshima fishing park, a salt factory, and was created.In this salt, Sweet bean paste wrapped in wheat Sweet bean paste 9 pieces / ¥660 calcium is included in richness and is the excellent & cream in rice cake article that the sweetness is felt slightly. Inside is sweetened bean paste 9 pieces / ¥660 containing beans intact and The exquisite harmony sweetness is moderate. The Marine Station Shop “Naoshima Rakuichi” which saltiness makes The mild taste by Shop 9:00~18:00 open year-round in sweet cream slightly which the salty (Town-Naoshima Tourism Association087-892-2299) taste worked faintly. “SOLASHIO” and the special products are sold. Activities EXPERIENCE Fishing Park Gotanji Swimming Beach There are a fixed pier, landing stage, a fish- ing raft, a fishing pond. -

Tokushima-Komatsushima Port Tourist Information
Tokushima-Komatsushima Port Tourist Information http://www.mlit.go.jp/kankocho/cruise/ Tokushima Local Gourmet 【① Tokushima Ramen】This local ramen features a brown pork bone and soy sauce soup, sukiyaki-style sweet and spicy pork ribs, and is topped with a raw egg.【②Fish Katsu】Minced fish is mixed with curry powder, covered in breadcrumbs, and deep-fried to make this fish katsu. The spicy taste and fragrance are top notch. 【③ Mametentama】Mametentama is a type of okonomiyaki that is packed with sweetly cooked red kidney beans. The salty-sweet taste is addicting. 【④Handa Somen】These hand-pulled noodles are thicker than average noodles and are characterized by their chewiness. These refreshing noodles especially taste great when paired with sudachi. Location/View ① ② Access Season Year-round Discover TOKUSHIMA Japan ③ ④ Related links https://discovertokushima.net/en/culture/cuisines/ Contact Us【Tourism Policy Division Tokushima Prefectural Government】 TEL:+81-88-621-2337 / E-MAIL: [email protected]/ Website: https://discovertokushima.net/ Awa Indigo Dyed Textiles Indigo is also known as "Japan Blue" . Known for its high quality, production of Awa indigo flourished during the Edo period (1603-1867). In Tokushima, the tradition of Awa indigo dyed textiles continues to this day. Indigo dyed textiles are said to have antibacterial, insect repellent, and moisture retention properties. Aside from buying ready-made items, there are also workshops and facilities that allow you to make your own Awa indigo goods. Location/View Access Season Year-round Discover TOKUSHIMA Japan Related links https://discovertokushima.net/en/culture/crafts/awa-indigo- dyed/ Contact Us【Tourism Policy Division Tokushima Prefectural Government】 TEL:+81-88-621-2337 / E-MAIL: [email protected]/ Website: https://discovertokushima.net/ Arudeyo Tokushima (Tokushima Souvenir and Tourism Plaza) Located on the 1st floor of the Awa Odori Kaikan at the foot of Mt. -

26 Kinoshita Chaen Geku-Mae Branch 28 Mother Fruit 25 Ise Mametoku 27 Cafe De Bon Vivant
Geku and Urban Area P. 32 Geku and Urban Area P. 32 25 Ise Mametoku 27 Cafe de Bon Vivant 和 発送 洋 席有 P有 発送 Tetora Ise Fuku Mame Mix is a sampler Financier with Powdered Rendaijigaki of beans with 8 different flavors such as Leaves is a financier (small French lobster and Ise tea.You can enjoy different cake) made with leaves of Rendaijigaki flavors little by little until you eat it up. (Japanese persimmons grown only in Ise City) and fermented butter. ☎0596-28-2860 1-1-29 Iwabuchi ☎0596-23-7170 営 9:00-16:00 Tetora Ise Fuku Mame Mix(1,296 yen for 20 small bags 18-30 Honmachi Financier with Powdered Rendaijigaki Leaves(170 yen each) 休 Mon.(Tue. if a public holiday falls on Mon.) ※Each bag contains 15 grams of beans.) 営 9:00-17:00(Mon.-Fri.)/9:00-18:00(Sat., Sun. 席 12 non-smoking seats and public holidays) P For 10 vehicles(This parking lot is also for 休 Open 365 days a year customers of the restaurant this store runs.) P No free parking lot (Nearby pay parking lot HP http://www.bonvivant1983.com available) ※Only baked confections available for delivery JR Sangu Line Kintetsu Yamada Line 22 Geku North ★ Iseshi 〒 21 French restaurant 37 201 Bon Vivant Honmachi 1 Ise Grand Shrine 37 (Geku) Inside of the store Ise Aosa Mame Shinju Mame ★ Mont Blanc(410 yen each) 32 (515 yen per bag) (515 yen per bag) 26 Kinoshita Chaen Geku-mae Branch 28 Mother Fruit 和 席有 洋 発送 Matcha Frozen is a frozen drink flavored Jingu Shiraishi Cookie is a cookie shaped with tasty green tea that contains no like a piece of o-shiraishi, white rounded artificial flavors.It is definitely rich in taste cobbles placed in the most sacred area of and very easy to drink. -

All New Menus
Syojin (v) Tasting menu 65 Sake pairings and Wine pairings are available Tar-Tar Chips Nibbles (Per piece) Edamame Spicy Citrus Dip Assorted vegetables (v) 1piecce 3.1 Avocado, yuzu garlic, jalapeño mayonnaise (v) Mixed vegetables tar-tar chips Avocado, yuzu garlic, jalapeño mayonnaise Toro fatty tuna 1piecce 4.6 Cold shuko apetisers Avocado, jalapeño mayonnaise Soba noodle salad Buckwheat soba noodle, mixed leaves, mixed cress, sesame vinaigrette Scottish salmon 1piecce 4.0 Avocado, chilli miso Hot shuko Dishes Yellowtail 1piecce 4.0 Tofu steamed mini burger bun Cherry tomato, tomato miso Avocado, jalapeño mayonnaise Nasu-miso double cooked aubergine, caramelised sweet miso with fresh truffle (£5.00 suppliment) Hand-dived Scottish scallop 1piecce 3.8 Avocado, spicy taramo sauce Agedashi Tofu Mushrooms, daikon, spring onion, ginger, shojin uma-dashi broth Snow crab 1piecce 4.1 Sushi Avocado, jalapeño mayonnaise Avocado tempura flakes and jalapeño mayo Creel caught native lobster 1piecce 4.6 Avocado, jalapeno mayonnaise Grilled shiitake mushroom truffle soy Seared Japanese Wagyu beef 1piecce 5.5 Grilled eringi mushroom yuzu soy Avocado, chilli miso Pickled daikon yuzu zest Miso Soups Assorted vegetable roll (6 pieces) ‘Shojin’ miso soup (v) 5.0 Dessert Seasonal mixed mushrooms Dark chocolate fondant tart vanilla ice cream Creel caught British native lobster miso soup 5.0 or Seasonal mixed mushrooms Selection of ice cream and sorbet 3 flavours Please ask your waiter for today's selection Bento petits fours du jour Some of our menu items contain allergens. There is a small risk that traces of these may be found in a number of our dishes. -

THE WESTIN TOKYO SEASONAL NEWS AUTUMN 2017 Sep.-Nov
THE WESTIN TOKYO SEASONAL NEWS AUTUMN 2017 Sep.-Nov. 153-8580 東京都目黒区三田1-4-1 Phone: 03-5423-7000 1-4-1 Mita Meguro-ku, Tokyo 153-8580 www.westin-tokyo.co.jp 写真はイメージです。特に指定がない限り、表示料金に別途税金とサービス料が加算されます。記載内容は予告なく変更に なる場合がございます。営業時間・最新の情報は、各レストランまたはホテルウェブサイトにてご確認いただけます。 Images are for illustrative purposes only. Prices are subject to tax and service charge unless otherwise stated. All content may change without notice. Please contact each restaurant or visit our website for opening hours and the latest information. シックで繊細、栗デザートの美術館 Elegant collection of delicate chestnut desserts インターナショナルレストラン ザ・テラス CHESTNUT 贅沢な秋のひととき International Restaurant THE TERRACE FOR A LUXURIOUS AUTUMN 03-5423-7778 DESSERT BUFFET マロン・デザートブッフェ MOMENT 毎年楽しみにされているお客様の多い、栗が主役のデザートブッフェ。 味わいや香り、食感。多彩な魅力を美しいデザートでお楽しみください。 This dessert buet starring chestnuts has now become a favorite of many guests visiting The Terrace during autumn every year. Welcoming you is an array of beautifully-decorated desserts featuring the attractive taste, smell, and texture of chestnuts. 10.2(Mon)~11.30(Thu) 平日限定 Weekdays only 15:00~17:00 大人 Adult ¥3,700 お子様 Child ¥1,850 SEASONAL NEWS AUTUMN 2017 01 02 WORLDWIDE CHOCOLATE DESSERT BUFFET -Chapter Two- ワールドチョコレート・デザートブッフェ ~第2章~ 五感で味わう、 チョコレートデザートの最新モード Chocolate desserts in the newest of styles, inspiring all five senses 芸術的なフォルム。芳醇なカカオの香り。なめら かな口どけ…。五感で楽しめる、チョコレートで 仕上げたデザート約30種類をお好きなだけお 楽しみいただけます。 The beautiful shapes, the rich aroma of cacao, and the smooth melting texture... Enjoy as much as you like of approximately 30 dierent chocolate desserts -

(12) Patent Application Publication (10) Pub. No.: US 2007/0212460 A1 Inoue Et Al
US 20070212460A1 (19) United States (12) Patent Application Publication (10) Pub. No.: US 2007/0212460 A1 Inoue et al. (43) Pub. Date: Sep. 13, 2007 (54) COMPOSITIONS CONTAINING SUCRALOSE Nov. 25, 1998 (JP)...................................... 1198-333948 AND APPLICATION THEREOF Nov. 30, 1998 (JP). ... 1998-34O274 Dec. 11, 1998 (JP) 1998-3534.89 (75) Inventors: Maki Inoue, Osaka (JP); Kazumi Iwai, Dec. 11, 1998 (JP) 1998-3534.90 Osaka (JP): Naoto Ojima, Osaka (JP); Dec. 11, 1998 (JP). ... 1998-3534.92 Takuya Kawai, Osaka (JP); Mitsumi Dec. 11, 1998 (JP) 1998-353495 Kawamoto, Osaka (JP); Shunsuke Dec. 11, 1998 (JP) 1998-353496 Kuribi, Osaka (JP); Miho Sakaguchi, Dec. 11, 1998 (JP). ... 1998-353498 Osaka (JP); Chie Sasaki, Osaka (JP); Dec. 11, 1998 (JP). ... 1998-3534.99 Kazuhito Shizu, Osaka (JP); Mariko Dec. 11, 1998 (JP). ... 1998-3535O1 Shinguryou, Osaka (JP); Kazutaka Dec. 11, 1998 (JP). ... 1998-353503 Hirao, Osaka (JP); Miki Fujii, Osaka Dec. 11, 1998 (JP). ... 1998-353504 (JP); Yoshito Morita, Osaka (JP); Dec. 11, 1998 (JP) 1998-353505 Nobuharu Yasunami, Osaka (JP); Dec. 11, 1998 (JP) 1998-353507 Junko Yoshifuji, Osaka (JP) Jan. 26, 1999 (JP)......................................... 1999-16984 Jan. 26, 1999 (JP)......................................... 1999-16989 Correspondence Address: Jan. 26, 1999 (JP)......................................... 1999-16996 SUGHRUE, MION, ZINN, MACPEAK & Apr. 6, 1999 (JP). ... 1999-158511 SEAS, PLLC Apr. 6, 1999 (JP). ... 1999-158523 2100 Pennsylvania Avenue, N.W. Apr. 6, 1999 (JP). ... 1999-158529 Washington, DC 20037-3202 (US) Apr. 6, 1999 (JP). ... 1999-158536 Apr. 6, 1999 (JP). ... 1999-158543 (73) Assignee: SAN-E GEN F.F.I., INC Apr. 6, 1999 (JP) 1999-158545 Apr. -
Pg 1 Macau Hide Message-EN New Photo Nov19 V1
Dearest Guest: Welcome to HIDE YAMAMOTO! We are so grateful that out of all the restaurants, we become your choice today. At HIDE YAMAMOTO, we present a multi-concept ambience of an international Japanese restaurant. Our food creation is far different of those fast-booming Japanese restaurants in Europe or the fusion Japanese restaurants in New York. The multi-concept starts with professional, skilled, and experienced chefs on each station. EDOMAE sushi chef at sushi counter, exceptional execution of Teppanyaki chefs, and innovative ideas from French-trained Robatayaki chefs. Each section has its own distinct way to present the food at its best, unparalleled even the top restaurant in Tokyo. I hope to share with you the food which I really enjoy. The honest and friendly service which you can only get here in HIDE YAMAMOTO. Be it a precious moment for yourself, with a loving partner, caring families or in the good company of your friends, let us make you feel that this place is an enjoyable place, like I do. Sincerely yours, Hidemasa Yamamoto 前前 蕎麥麵配法國魚子醬 Buckwheat Soba with Caviar 菜菜 C O L D DIS H 綠蔬菜沙律 綠蔬菜沙律 三種醬料可選 日式醬油酸汁或洋蔥胡椒汁或西京味噌汁 95 Seasonal Green Vegetable Salad Choice of Dressing: Soy Vinegar, Onion Pepper, Saikyo Miso 蒸油甘魚配蕪菁沙律 150 Steamed Yellowtail with Turnip Salad 蒸油甘魚配蕪菁沙律 酥炸海蝦配咖喱鹽 100 Deep-fried Jack-Knife Prawn with Curry Salt 季節三小前菜 180 Trio Appetizers 酥炸海蝦配咖喱鹽 蕎麥麵配法國魚子醬 170 Buckwheat Soba with Caviar ※ 時令食材會因季節而作出更換。請告知您的服務員關於任何食物過敏或餐飲限制。 所有價格為澳門幣並需加收10%服務費。 ※ Seasonal ingredients may change according to availability. Please inform our service staff if you have any food allergies or dietary requirements. -

Il Packaging Tradizionale Giapponese
Corso di Laurea magistrale in Lingue e civiltà dell'Asia e dell'Africa mediterranea Tesi di Laurea Il packaging tradizionale giapponese Relatore Ch.ma Prof.ssa Silvia Vesco Correlatore Ch.ma Prof.ssa Sabrina Rastelli Laureanda Alice Misciagna Matricola 815746 Anno Accademico 2013 / 2014 INDICE 「日本の伝統パッケージ」 .................................................................................................................. i INTRODUZIONE ................................................................................................................................... 1 CAPITOLO 1. Caratteristiche e materiali. ............................................................................................ 13 1.1 Legno (木 ki) .......................................................................................................................... 13 1.2 Bambù (竹 take) ..................................................................................................................... 29 1.3 Bambù nano (笹 sasa) ........................................................................................................... 45 1.4 Paglia (藁 wara) ..................................................................................................................... 50 1.5 Ceramica (土 tsuchi) .............................................................................................................. 60 1.6 Carta (紙 kami) ...................................................................................................................... 76 Alcune tipologie