Perforated Blind Pouch: an Unusual Late Complication Following Lateral Anastomosis After a Right Hemicolectomy
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Recent Advances in Surgery the Blind-Loop Syndrome After Gastric
Recent advances in surgery Conducted by ALFRED BLALOCK, M.D. The blind-loop syndrome after gastric operations ince the earliest reports by Whitees time, several examples of the blind-loop ANATOMIC CONFIGURATION OF BLIND LOOPS Various examples of gastrointestinal blind and Wangensteen;O a side-arm loop has been employed as in Fig. 1, A, in which the loop is arranged so as to be self-filling. The side loop arrangement is the one which most 849 850 Recent advances in surgery Surgery ;.$ Nouernbcr 1961 & ad cumulated from observations on lesions at differing sites in the gastrointestinal tract. The principles involved apply, with varia- tions, to blind loops at all levels. The best- known feature of the blind-loop syndrome is megaloblastic anemia, which is due to dis- ruption of vitamin BI2 absorption. Normally, dietary vitamin B12 (Castle's extrinsic fac- tor) is absorbed after an incompletely under- stood interaction with intrinsic factor (Fig. 3, A), a mucoprotein secreted by the gastric rnu~osa.*~In man, the principal site of B,, absorption is the ileum.7* Vitamin B,, deficiency can develop by a number of alternative mechanisms. Rarely loops which have caGsed the blind-loop- syndrome.- is there dietary deficiency of this nutritional A, Anastomosis with formation of a self-filled stagnant loop, B, with jejunal diverticulosis, C, factor. Commonly, as in pernicious anemia with intestinal strictures, D, after enteroenteros- (Fig. 3, B) or after total gastrectomy,Sgthere tomies or fistulas, and E, after gastric operation. is absent intrinsic factor due to gastric atrophy or the absence. of the stomach, re- resembles a blind loop which develops after spectively. -

Long-Term Outcome After Neonatal Meconium Obstruction
Long-term Outcome After Neonatal Meconium Obstruction Julie R. Fuchs, MD, and Jacob C. Langer, MD ABSTRACT. Objective. It is unclear whether children meconium ileus and those undergoing resection or enter- with cystic fibrosis (CF) who present with neonatal ostomy. Patients with meconium obstruction who do not meconium ileus have a different long-term outcome from have CF have an excellent long-term prognosis. This those presenting later in childhood with pulmonary com- information will be useful in counseling the families of plications or failure to thrive. We examined a cohort of infants presenting with neonatal meconium obstruction. patients with meconium ileus, and compared their long- Pediatrics 1998;101(4). URL: http://www.pediatrics.org/ term outcome with children who had CF without meco- cgi/content/full/101/4/e7; cystic fibrosis, meconium ileus, nium ileus and neonates who had meconium obstruction meconium plug syndrome. without CF (meconium plug syndrome). Study Design. Comparative study using retrospective and follow-up interview data. ABBREVIATION. CF, cystic fibrosis. Patients. Group 1 consisted of 35 surviving CF pa- tients who presented with meconium ileus between 1966 econium obstruction in the neonate is a and 1992. Two control groups were also studied: 35 age- spectrum of disease that includes meco- and sex-matched CF patients without meconium ileus 1 (group 2), and 12 infants presenting with meconium plug Mnium ileus and meconium plug syndrome. syndrome during the same time period (group 3). Meconium ileus is characterized by extremely viscid, Outcome Measures. Pulmonary, gastrointestinal, nu- protein-rich inspissated meconium causing terminal tritional, and functional status were reviewed, and sur- ileal obstruction, and accounts for approximately gical complications were recorded. -

Diagnostic Codes
DISEASES OF THE DIGESTIVE SYSTEM DISEASES OF ORAL CAVITY, SALIVARY GLANDS AND JAWS (520 - 529.9) 520 DISORDERS OF TOOTH DEVELOPMENT AND ERUPTION 520.0 ANODONTIA 520.1 SUPERNUMERARY TEETH 520.2 ABNORMALITIES OF SIZE AND FORM 520.3 MOTTLED TEETH 520.4 DISTURBANCES OF TOOTH FORMATION 520.5 HEREDITARY DISTURBANCES IN TOOTH STRUCTURE, NOT ELSEWHERE CLASSIFIED 520.6 DISTURBANCES IN TOOTH ERUPTION 520.7 TEETHING SYNDROME 520.8 OTHER DISORDERS OF TOOTH DEVELOPMENT 520.9 UNSPECIFIED 521 DISEASES OF HARD TISSUES OF TEETH 521.0 DENTAL CARIES 521.1 EXCESSIVE ATTRITION 521.2 ABRASION 521.3 EROSION 521.4 PATHOLOGICAL RESORPTION 521.5 HYPERCEMENTOSIS 521.6 ANKYLOSIS OF TEETH 521.7 POSTERUPTIVE COLOUR CHANGES 521.8 OTHER DISEASES OF HARD TISSUES OF TEETH 521.9 UNSPECIFIED 522 DISEASES OF PULP AND PERIAPICAL TISSUES 522.0 PULPITIS 522.1 NECROSIS OF THE PULP 522.2 PULP DEGENERATION 522.3 ABNORMAL HARD TISSUE FORMATION IN PULP 522.4 ACUTE APICAL PERIODONTITIS OF PULPAL ORIGIN 522.5 PERIAPICAL ABSCESS WITHOUT SINUS 522.6 CHRONIC APICAL PERIODONTITIS 522.7 PERIAPICAL ABSCESS WITH SINUS 522.8 RADICULAR CYST 522.9 OTHER AND UNSPECIFIED 523 GINGIVAL AND PERIODONTAL DISEASES 523.0 ACUTE GINGIVITIS 523.1 CHRONIC GINGIVITIS 523.2 GINGIVAL RECESSION 523.3 ACUTE PERIODONTITIS 523.4 CHRONIC PERIODONTITIS 523.5 PERIODONTOSIS 523.6 ACCRETIONS ON TEETH 523.8 OTHER PERIODONTAL DISEASES 523.9 UNSPECIFIED 524 DENTOFACIAL ANOMALIES, INCLUDING MALOCCLUSION 524.0 MAJOR ANOMALIES OF JAW SIZE 524.1 ANOMALIES OF RELATIONSHIP OF JAW TO CRANIAL BASE 524.2 ANOMALIES OF DENTAL ARCH -

Dysfunction of the Continent Ileostomy: Clinical Features and Bacteriology*
Gut: first published as 10.1136/gut.24.3.193 on 1 March 1983. Downloaded from Gut, 1983, 24, 193-201 Dysfunction of the continent ileostomy: clinical features and bacteriology* DARLENE G KELLY, S F PHILLIPS. K A KELLY, W M WEINSTEIN, AND MARY J R GILCHRIST From the Gastroenterology Unit and Department of Laboratorv Medicine, Mayo Clinic and Mayo Foundation, Rochester, Minnesota, and the Departmlent of Medicine, University of California, Los Angeles, California, USA SUMMARY The pathogenesis and treatment of dysfunction of the continent ileostomy was investigated in 12 patients, five of whom had asymptomatic malabsorption and seven of whom had acute complaints. The number of anaerobic bacteria in jejunal aspirates was increased in patients with pouch malfunction (range 103 to 108/g aspirate), but the microbiology of ileal effluent and the morphology of the ileal mucosa could not be correlated with dysfunction. Bile acid breath tests and lactose tolerance tests were not, however, reliable indicators of jejunal bacterial overgrowth. The symptoms, the malabsorption, and the number of jejunal and ileal anaerobic bacteria decreased in patients during treatment with metronidazole, implicating overgrowth of anaerobic bacterial flora in the pathogenesis of the syndrome. The continent ileostomy (ileal pouch), devised by vitamin B12,9 12 proliferation of anaerobic bacteria http://gut.bmj.com/ Kock,' has been used as an alternative to con- in the pouch,9 inflammation in the pouch,7 ventional ileostomy for selected patients with incontinence,"' difficulty with intubation,1' and ulcerative colitis and familial polyposis.2-5 The bloody discharge.5 We previously reported reservoir functions satisfactorily in most patients, diarrhoea with some features of malabsorption in but malfunction of the 'nipple valve' may occur in up approximately one-third of asymptomatic patients to a third of patients and lead to incontinence. -

A Rare Case of Multiple Perforated Jejunal Diverticulae - Case Report
International Journal of Recent Trends in Science And Technology, ISSN 2277-2812 E-ISSN 2249-8109, Volume 10, Issue 3, 2014 pp 458-460 A Rare Case of Multiple Perforated Jejunal Diverticulae - Case Report B. Easwaran 1, G. P. Sekar 2, K. Senthil Kumaran 3, B. Selvaraj 4 1Professor and HOD, 2,4 Associate Professor, 3Assisstant Professor, Department of Surgery, SVMCH, Pondicherry, INDIA. *Corresponding Address: [email protected] Case report Abstract: Jejunal diverticula are very rare with an incidence of less rate 70/Min and BP was 100/60 mm HG. Systemic 1 than 0.5% and are usually asymptomatic. The etiology remains examination was normal. The abdomen was soft, unclear. Since they arise from herniation of the mucosa and distended and tenderness was present in all quadrants. A submucosa through a weak portion of the bowel they are classified as false diverticula of the pulsion type. Complications occur in 10 3 hours later guarding and rigidity also developed to 30% of patients (Ref. 22 ). They are chronic abdominal pain, Blind .clinically free fluid was also present. The bowel sounds loop syndrome, malabsorption, steatorrhoea, megaloblastic anemia, were sluggish. There was tenderness on per rectal hemorrhage, diverticulitis, obstruction, abscess formation, examination. Lab tests revealed impaired renal profile intussusception, volvulus 23 , small bowel obstruction due to 24,25,26,27 (urea 130 mg/d L; creatinine 4.5mg/d L) .Other blood enteroliths and rarely perforation. Jejunal diverticula have a tests were normal. Abdominal x-ray showed air under higher rate of complication than other small bowel diverticula 22 . Diagnosing complicated acute jejunal diverticulosis based on right dome of diaphragm, dilated small bowel loops. -

190.18 - Serum Iron Studies
Medicare National Coverage Determinations (NCD) Coding Policy Manual and Change Report (ICD-10-CM) 190.18 - Serum Iron Studies HCPCS Codes (Alphanumeric, CPT AMA) Code Description 82728 Ferritin 83540 Iron 83550 Iron Binding capacity 84466 Transferrin ICD-10-CM Codes Covered by Medicare Program The ICD-10-CM codes in the table below can be viewed on CMS’ website as part of Downloads: Lab Code List, at http://www.cms.gov/Medicare/Coverage/CoverageGenInfo/LabNCDsICD10.html Code Description A01.00 Typhoid fever, unspecified A01.01 Typhoid meningitis A01.02 Typhoid fever with heart involvement A01.03 Typhoid pneumonia A01.04 Typhoid arthritis A01.05 Typhoid osteomyelitis A01.09 Typhoid fever with other complications A01.1 Paratyphoid fever A A01.2 Paratyphoid fever B A01.3 Paratyphoid fever C A01.4 Paratyphoid fever, unspecified A02.0 Salmonella enteritis A02.1 Salmonella sepsis A02.20 Localized salmonella infection, unspecified NCD 190.18 January 2021 Changes ICD-10-CM Version – Red Fu Associates, Ltd. January 2021 1 Medicare National Coverage Determinations (NCD) Coding Policy Manual and Change Report (ICD-10-CM) Code Description A02.21 Salmonella meningitis A02.22 Salmonella pneumonia A02.23 Salmonella arthritis A02.24 Salmonella osteomyelitis A02.25 Salmonella pyelonephritis A02.29 Salmonella with other localized infection A02.8 Other specified salmonella infections A02.9 Salmonella infection, unspecified A04.0 Enteropathogenic Escherichia coli infection A04.1 Enterotoxigenic Escherichia coli infection A04.2 Enteroinvasive Escherichia -

Progress Report Small Bowel Resection and Gastric Acid Hypersecretion
Gut: first published as 10.1136/gut.15.3.229 on 1 March 1974. Downloaded from Cut, 1974, 15, 229-238 Progress report Small bowel resection and gastric acid hypersecretion Resection of a large segment of small intestine in the dog is associated with an increase in gastric acid secretion1-'8. However, the occurrence of gastric acid hypersecretion after small bowel resection in man remains in doubt. The benign postoperative course of patients who have undergone an ex- tensive intestinal resection after having had a previous gastric resection was first observed by Craig and Stewart (1960)19. Since then, the beneficial effect on survival of measures to control gastric acid secretion after extensive small bowel resection has been well documented4'20. An acute syndrome consisting of the aspiration of copious quantities of acid from the stomach after small bowel resection has been reported in man1421. The copious aspirate usually subsides in the first few weeks after surgery. There are few reports of long-term changes in acid secretion after small bowel resection or exclusion. A low pH of the jejunal contents in six patients of Krone, Theodore, Sleisinger, and Jeffries22 suggests that hyper- secretion exists after small bowel resection. However, the work of Shibata, McKenzie, and Long23, Salmon and Wright24, and Buchwald and Varco25 has not confirmed the increase in acid secretion in patients undergoing small http://gut.bmj.com/ bowel bypass. In man, therefore, the circumstantial evidence that is available suggests that there is a syndrome ofhypersecretion after small bowel resection but the few studies of acid secretion which have been reported have not verified the presence of this syndrome. -

Emerging Parasitic Infection Mimicking Functional Bowel Disease Javed Yakoob Aga Khan University, [email protected]
eCommons@AKU Section of Gastroenterology Department of Medicine May 2010 Emerging Parasitic Infection Mimicking Functional Bowel Disease javed Yakoob Aga Khan University, [email protected] Wasim Jafri Aga Khan University, [email protected] Mohammad Asim Beg Aga Khan University Z Abbas Aga Khan University, [email protected] shagufta Naz, Aga Khan University See next page for additional authors Follow this and additional works at: http://ecommons.aku.edu/ pakistan_fhs_mc_med_gastroenterol Part of the Gastroenterology Commons Recommended Citation Yakoob, j., Jafri, W., Beg, M. A., Abbas, Z., Naz,, s., Islam, M., Khalid, A., Khan, R., Ahmad, Z. (2010). Emerging Parasitic Infection Mimicking Functional Bowel Disease. Gastroenterology, 138(5 (supplement 1)), S-587-S-588. Available at: http://ecommons.aku.edu/pakistan_fhs_mc_med_gastroenterol/118 Authors javed Yakoob; Wasim Jafri; Mohammad Asim Beg; Z Abbas; shagufta Naz,; Muhammad Islam; A. B. Khalid; Rustam Khan; and Zubair Ahmad This article is available at eCommons@AKU: http://ecommons.aku.edu/pakistan_fhs_mc_med_gastroenterol/118 complex containing both protein and RNA molecules (i.e., a ribonucleoprotein complex) T1828 and small nuclear ribonuclear polypeptide A were present in high titers in serum that contained anti-enteric neuronal antibodies in our neuronal assays. Our Invitrogen®ProtoAr- Fructose and Lactose Intolerance: Association With Atopy and Extra-GI ray assay did not find these antibodies in serum in which our immunostaining assay found Symptoms no anti-enteric neuronal antibodies. Moderate levels of anti-ro 52000 MW antibody were Clive H. Wilder-Smith, Andrea Materna present also in IBS serum. Anti-ro 52000 is a known biomarker for Sjögren's syndrome. INTRODUCTION: Sugar intolerances are common in patients with functional bowel dis- Conclusion: The gut does not work well when the enteric nervous system (ENS) is not orders (FBD), although the prevalence of sugar malabsorption in not dissimilar to that in working well. -

Progress Report Diarrhoea: Mechanisms and Treatment
Gut: first published as 10.1136/gut.12.12.1021 on 1 December 1971. Downloaded from Gut, 1971, 12, 1021-1036 Progress report Diarrhoea: Mechanisms and treatment Diarrhoea can usefully be considered as a disturbance in the balance between the mechanisms controlling secretion and absorption of water resulting in an excess loss of water in the faeces. In this economy, the quantity of water in food and drink is of little importance,' for compulsive water drinkers, who may drink 10 litres of water a day, do not develop diarrhoea. Flow of water across the gut occurs mainly to maintain isotonicity with the plasma in a rapidly changing chemical environment. Thus intraluminal digestion increases the osmotic attraction of water into the bowel, while absorption or secretion of solute is accompanied by absorption or secretion of water.2'3 In the upper small bowel the absorptive forces are augmented by active transport of sugars and amino acids coupled with rather a weak sodium pump. In the ileum and colon a powerful sodium pump is capable of transporting sodium isotonically against large electrochemical gradients.4'5'6 These transport systems are closely integrated with the transfer of a number of other substances such as the interchange of chloride for bicarbonate.7'8 Although these transfers may not play a direct part in drawing water out of the intestinal lumen, the pathways are so interdependent that a severe dis- turbance of any of them may result in diarrhoea.9 The study of the humoral, electrochemical, and structural factors which control these processes has led to useful advances in the treatment of diarrhoea, although our understanding http://gut.bmj.com/ of the underlying mechanisms remains fragmentary. -
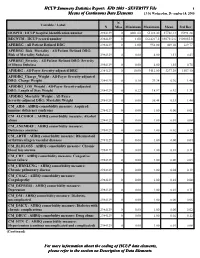
Stats KID 2003 Severity File
HCUP Summary Statistics Report: KID 2003 - SEVERITY File 1 Means of Continuous Data Elements 13:36 Wednesday, December 14, 2005 N Variable / Label N Miss Minimum Maximum Mean Std Dev HOSPID : HCUP hospital identification number 2984129 0 4001.00 55188.00 27728.13 15991.24 RECNUM : HCUP record number 2984129 0 1.00 6614217.0 3307312.6 1909855.8 APRDRG : All Patient Refined DRG 2984129 0 1.00 956.00 469.20 229.77 APRDRG_Risk_Mortality : All Patient Refined DRG: Risk of Mortality Subclass 2984129 0 0.00 4.00 1.11 0.43 APRDRG_Severity : All Patient Refined DRG: Severity of Illness Subclass 2984129 0 0.00 4.00 1.55 0.75 APSDRG : All-Payer Severity-adjusted DRG 2984129 0 10.00 9112.00 3277.30 1807.40 APSDRG_Charge_Weight : All-Payer Severity-adjusted DRG: Charge Weight 2984129 0 0.10 29.24 0.76 1.48 APSDRG_LOS_Weight : All-Payer Severity-adjusted DRG: Length of Stay Weight 2984129 0 0.22 18.97 0.92 1.31 APSDRG_Mortality_Weight : All-Payer Severity-adjusted DRG: Mortality Weight 2984129 0 0.00 36.44 0.33 1.46 CM_AIDS : AHRQ comorbidity measure: Acquired immune deficiency syndrome 2984129 0 0.00 1.00 0.00 0.01 CM_ALCOHOL : AHRQ comorbidity measure: Alcohol abuse 2984129 0 0.00 1.00 0.01 0.08 CM_ANEMDEF : AHRQ comorbidity measure: Deficiency anemias 2984129 0 0.00 1.00 0.02 0.15 CM_ARTH : AHRQ comorbidity measure: Rheumatoid arthritis/collagen vascular diseases 2984129 0 0.00 1.00 0.00 0.04 CM_BLDLOSS : AHRQ comorbidity measure: Chronic blood loss anemia 2984129 0 0.00 1.00 0.01 0.11 CM_CHF : AHRQ comorbidity measure: Congestive heart failure -

8 (D Husbandry Has Gained Momentum
EVOLVING KNOWLEDGE OF DIGESTIVE DYSFUNCTION H.J.Breukink and R.Kuiper, Department of Large Animal Medicine (Q) and Nutrition, Veterinary Faculty, University of Utrecht, n Yalelaan 16, 3508 TD Utrecht, The Netherlands, 0 "'O ~ INTRODUCTION ..... (JQg Digestive dysfunction or indigestion has been a subject of ► great interest for every bovine practitioner since dairy 8 (D husbandry has gained momentum. In the dairy cow most attention .....'"i . has been drawn towards the rumen since the outcome of the (") digestive processes in the rumen for a great part determine § the productivity of the cow. Recently nutritionists have investigated feeding methods aimed at avoiding ruminal degra 00► 00 dation, in order to cope with the enormous demand for glucose 0 and proteins in a dairy cow producing more than 40 liters of (")..... milk per day . Under those nutritional regimes the outcome of the microbial fermentation however remains of great importan a...... 0 ce. Even small disturbances in the microbial digestion can ::::s have an immense impact on the produ~tion, and may, when occur 0 ring in the second half of the lactation period, ruin the I-!; total performance in that lactation period. It is therefore td important to recognise and classify any indigestion as early 0 as possible. The same is true for digestive disturbances in <s · replacement stock. These animals have to experience an undis (D turbed growth during their first two years of life. Only in ~ that case they are able to respond to the present tremendous ~ demands for production. After a short introduction into the (") classification of the digestive disturbances in the ruminating .-+-..... -

CSHCS Qualifying Diagnoses Effective 01/01/2021
CSHCS Qualifying Diagnoses Effective 01/01/2021 Medical Review ICD-10 Code Description Period Code End Date A1854 Tuberculous iridocyclitis 2 A507 Late congenital syphilis, unspecified 5 A925 Zika virus disease 2 B20 Human immunodeficiency virus [HIV] disease 5 B259 Cytomegaloviral disease, unspecified 5 B393 Disseminated histoplasmosis capsulati 2 B399 Histoplasmosis, unspecified 2 B900 Sequelae of central nervous system tuberculosis 5 B902 Sequelae of tuberculosis of bones and joints 3 B909 Sequelae of respiratory and unspecified tuberculosis 3 B91 Sequelae of poliomyelitis 5 B941 Sequelae of viral encephalitis 5 B942 Sequelae of viral hepatitis 2 B948 Sequelae of other specified infectious and parasitic diseases 2 C029 Malignant neoplasm of tongue, unspecified 5 C039 Malignant neoplasm of gum, unspecified 5 C051 Malignant neoplasm of soft palate 5 C080 Malignant neoplasm of submandibular gland 5 C089 Malignant neoplasm of major salivary gland, unspecified 5 C118 Malignant neoplasm of overlapping sites of nasopharynx 5 C119 Malignant neoplasm of nasopharynx, unspecified 5 C169 Malignant neoplasm of stomach, unspecified 5 C179 Malignant neoplasm of small intestine, unspecified 5 C188 Malignant neoplasm of overlapping sites of colon 5 C189 Malignant neoplasm of colon, unspecified 5 C19 Malignant neoplasm of rectosigmoid junction 5 C210 Malignant neoplasm of anus, unspecified 5 C220 Liver cell carcinoma 5 C222 Hepatoblastoma 5 C223 Angiosarcoma of liver 5 C224 Other sarcomas of liver 5 C227 Other specified carcinomas of liver 5 C228 Malignant