3-Carosi 979[Cybium 2017, 411]11-23.Indd
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Biogeography and Evolution of the Carassius Auratus-Complex in East
Takada et al. BMC Evolutionary Biology 2010, 10:7 http://www.biomedcentral.com/1471-2148/10/7 RESEARCH ARTICLE Open Access Biogeography and evolution of the Carassius auratus-complex in East Asia Mikumi Takada1,2*, Katsunori Tachihara1, Takeshi Kon2, Gunji Yamamoto2, Kei’ichiro Iguchi3, Masaki Miya4, Mutsumi Nishida2 Abstract Background: Carassius auratus is a primary freshwater fish with bisexual diploid and unisexual gynogenetic triploid lineages. It is distributed widely in Eurasia and is especially common in East Asia. Although several genetic studies have been conducted on C. auratus, they have not provided clear phylogenetic and evolutionary descriptions of this fish, probably due to selection bias in sampling sites and the DNA regions analysed. As the first step in clarifying the evolutionary entity of the world’s Carassius fishes, we attempted to clarify the phylogeny of C. auratus populations distributed in East Asia. Results: We conducted a detailed analysis of a large dataset of mitochondrial gene sequences [CR, 323 bp, 672 sequences (528 sequenced + 144 downloaded); CR + ND4 + ND5 + cyt b, 4669 bp in total, 53 sequences] obtained from C. auratus in East Asia. Our phylogeographic analysis revealed two superlineages, one distributed mainly among the Japanese main islands and the other in various regions in and around the Eurasian continent, including the Ryukyus and Taiwan. The two superlineages include seven lineages with high regional specificity that are composed of endemic populations indigenous to each region. The divergence time of the seven lineages was estimated to be 0.2 million years ago (Mya) by a fossil-based method and 1.0-1.9 Mya by the molecular clock method. -
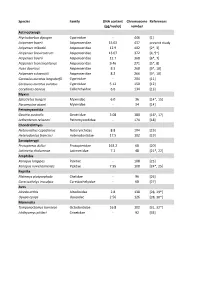
Table S2.Xlsx
Species Family DNA content Chromosome References (pg/nuclei) number Actinopterygii Ptychobarbus dipogon Cyprinidae ‐ 446 [1] Acipenser baerii Acipenseridae 15.02 437 present study Acipenser mikadoi Acipenseridae 12.9 402 [2*, 3] Acipenser brevirostrum Acipenseridae 13.07 372 [4, 5*] Acipenser baerii Acipenseridae 12.7 368 [6*, 7] Acipenser transmontanus Acipenseridae 9.46 271 [5*, 8] Huso dauricus Acipenseridae 8.3 268 [9*, 10] Acipenser schrenckii Acipenseridae 8.2 266 [9*, 10] Carassius auratus langsdorfii Cyprinidae ‐ 204 [11] Carassius auratus auratus Cyprinidae 5.12 150 [12] Corydoras aeneus Callichthyidae 6.6 134 [13] Myxini Eptatretus burgeri Myxinidae 6.0 36 [14*, 15] Paramyxine atami Myxinidae ‐ 34 [14] Petromyzontida Geotria australis Geotriidae 3.08 180 [16*, 17] Lethenteron reissneri Petromyzontidae ‐ 174 [18] Chondrichthyes Notorynchus cepedianus Notorynchidae 8.8 104 [19] Heterodontus francisci Heterodontidae 17.5 102 [19] Sarcopterygii Protopterus dolloi Protopteridae 163.2 68 [20] Latimeria chalumnae Latimeriidae 7.2 48 [21*, 22] Amphibia Xenopus longipes Pipidae ‐ 108 [23] Xenopus ruwenzoriensis Pipidae 7.95 108 [24*, 25] Reptilia Platemys platycephala Chelidae ‐ 96 [26] Carettochelys insculpta Carettochelyidae ‐ 68 [27] Aves Alcedo atthis Alcedinidae 2.8 138 [28, 29*] Upupa epops Upupidae 2.56 126 [28, 30*] Mammalia Tympanoctomys barrerae Octodontidae 16.8 102 [31, 32*] Ichthyomys pittieri Cricetidae ‐ 92 [33] References 1. Yu XY, Yu XJ. A schizothoracin fish species, Diptychus dipogon, with very high number of chromosomes Chrom Inform Serv. 1990;48:17-8. 2. Zhou H, Fujimoto T, Adachi S, Yamaha E, Arai K. Genome size variation estimated by flow cytometry in Acipenser mikadoi, Huso dauricus in relation to other species of Acipenseriformes. -

Chondrostoma Nasus) Ecological Risk Screening Summary
Common Nase (Chondrostoma nasus) Ecological Risk Screening Summary U.S. Fish & Wildlife Service, April 2020 Revised, April 2020 Web Version, 2/8/2021 Organism Type: Fish Overall Risk Assessment Category: High Photo: André Karwath. Licensed under CC BY-SA 2.5. Available: https://commons.wikimedia.org/wiki/File:Chondrostoma_nasus_(aka).jpg#file. (April 2020). 1 Native Range and Status in the United States Native Range From Froese and Pauly (2021): “Europe: Basins of Black (Danube, Dniestr, South Bug and Dniepr drainages), southern Baltic (Nieman, Odra, Vistula) and southern North Seas (westward to Meuse). […] Asia: Turkey.” Status in the United States No information on occurrence, status, sale or trade in the United States was found. Chondrostoma nasus falls within Group I of New Mexico’s Department of Game and Fish Director’s Species Importation List (New Mexico Department of Game and Fish 2010). Group I species “are designated semi-domesticated animals and do not require an importation permit.” With the added restriction of “Not to be used as bait fish.” 1 Means of Introductions in the United States No introductions have been reported in the United States. Remarks Although the accepted and most used common name for Chondrostoma nasus is “Common Nase”, it appears that the simple name “Nase” is sometimes used to refer to C. nasus (Zbinden and Maier 1996; Jirsa et al. 2010). The name “Sneep” also occasionally appears in the literature (Irz et al. 2006). 2 Biology and Ecology Taxonomic Hierarchy and Taxonomic Standing From Fricke et al. (2020): -

Polyphyletic Origin of Ornamental Goldfish
Food and Nutrition Sciences, 2015, 6, 1005-1013 Published Online August 2015 in SciRes. http://www.scirp.org/journal/fns http://dx.doi.org/10.4236/fns.2015.611104 Polyphyletic Origin of Ornamental Goldfish Aleksandr V. Podlesnykh1, Vladimir A. Brykov1,2, Lubov A. Skurikhina1 1Far Eastern Branch of the Russian Academy of Sciences, A. V. Zhirmunsky Institute of Marine Biology, Vladivostok, Russia 2School of Natural Sciences, Far Eastern Federal University, Vladivostok, Russia Email: [email protected] Received 6 May 2015; accepted 17 August 2015; published 20 August 2015 Copyright © 2015 by authors and Scientific Research Publishing Inc. This work is licensed under the Creative Commons Attribution International License (CC BY). http://creativecommons.org/licenses/by/4.0/ Abstract Mitochondrial DNA fragment of cytb was compared in all species Carassius auratus complex and three forms of ornamental goldfish. It is shown that the phylogenetic relationships between com- plex species correspond to the existing views, based on mtDNA data and geographical distribution. All forms of ornamental goldfish have a monophyletic origin from Chinese goldfish C. auratus au- ratus. The analysis showed that three nuclear genes (rps7, GH1 and Rh) in the two forms of orna- mental goldfish (Oriental twintail goldfish and Chinese Ranchu) were almost identical C. auratus auratus genes, while all three gene in another more simple form of goldfish (common goldfish) were highly homologous to carp Cyprinus carpio nuclear genes. The obtained data suggested that in the history of aquarium goldfish breeding occurred the stage of distant hybridization between goldfish and common carp. Subsequently, the nuclear genomes of some ornamental forms could be enriched by goldfish genes (a relatively recent form as Oriental twintail goldfish and Chinese Ranchu) or common carp genes (the simplest and most ancient forms like common goldfish) as a result of multidirectional breeding and selection of aquarium goldfish various forms. -

Electrophoretic Studies of Diploid, Triploid, and Tetraploid Forms of the Japanese Silver Crucian Carp, Carassius Auratus Langsdorfii
Japan.J.Ichthyol. 魚 類 学 雑 誌 40(1):65-75,1993 40(1):65-75,1993 Electrophoretic Studies of Diploid, Triploid, and Tetraploid Forms of the Japanese Silver Crucian Carp, Carassius auratus langsdorfii Yasushi Shimizu,1,2 Takashi Oshiro1 and Mitsuru Sakaizumi2,31 Department of Aquatic Biosciences, Tokyo University of Fisheries, 4-5-7 Konan, Minato-ku, Tokyo 108, Japan 2Department of Experimental Animal Science, The Tokyo Metropolitan Institute of Medical Science, 3-18-22 Honkomagome, Bunkyo-ku, Tokyo 113, Japan 3Present address: Department of Biology, College of General Education, Niigata University, 2-8050 Ikarashi, Niigata 950-21, Japan (Received October 29, 1992; in revisedform February 8, 1993; accepted March 22, 1993) Abstract The diploid-polyploid complex in the Japanese silver crucian carp (ginbuna), Carassius auratus langsdorfii, includes a diploid bisexual form, a triploid gynogenetic form and a tetraploid gynogenetic form. However, the origin of the polyploids remains to be clarified. Flow cytometric and electrophoretic analyses of Japanese crucian carp demonstrated that all the individuals with a two-banded pattern for an AMY-2 gene were polyploid, and 90% of the polyploids gave such a two-banded pattern. One of the two bands was not found in diploid subspecies in Japan but was present in both Korean crucian carp and goldfish. The diploid subspecies, kinbuna (C.auratus subsp.), which is distributed along the Pacific coast of eastern Japan, generated a diagnostic band of phosphoglucomutase. This band was also found in the polyploid "ginbuna" from the same region, but was observed in neither triploids nor diploids from other regions. -

Catalog of Fishes Queries April 2017 Dennis Polack Fishwisepro Lineolatus, Apogon Rüppell [W
Catalog of Fishes Queries April 2017 Dennis Polack Fishwisepro lineolatus, Apogon Rüppell [W. P. E. S.] 1829:47, Pl. 12 (fig. 1) [Atlas zu der Reise im nördlichen Africa. Fische des Rothen Meeres; ref. 3843] Massawa, Eritrea, Red Sea. •Permanently invalid, preoccupied by Apogon lineolatus Cuvier 1828 -- (T. Fraser, pers. comm. 9/2000). •Synonym of Archamia lineolata (Cuvier 1828) -- (T. Fraser, pers. comm. 9/2000). Current status: Synonym of Archamia lineolata (Cuvier 1828). Apogonidae: Apogoninae. Habitat: marine. Taeniamia lineolata : maculatus, Liparis Malm [A. W.] 1865:412 [Förhandlingar vid de Skandinaviske Naturforskarnes. v. 9; ref. 17596] Bukn, Bohüslän Island. No types known. Syntypes: NHMG 963 (1), 1233 (1) •Synonym of Liparis montagui (Donovan 1804) -- (Chernova 1991:28 [ref. 23263], Chernova et al. 2004:27 [ref. 27592], Chernova 2008:832 [ref. 30236]). Current status: Synonym of Liparis montagui (Donovan 1804). Liparidae. Habitat: marine. This record appears to be marked as not available but no mention of in synonymy. : crosnieri, Chirolophius (Pyrenophorus) Le Danois [Y.] 1975:77, Figs. 52, 59 [Mémoires du Muséum National d'Histoire Naturelle Serie A Zoologie v. 91; ref. 2732] Of northwestern Madagascar, 12°44'08"S, 48°10'06"E, depth 563-570 meters. Holotype: MNHN 1973-0023. Paratypes: MNHN 1973-0024 to 0026 (1, 1, 1). Type catalog: Pietsch et al. 1986:135 [ref. 6339]. •Synonym of Lophiodes insidiator (Regan 1921) -- (Caruso 1981:527 [ref. 5169], Caruso 1986:364 [ref. 6290]). Current status: Synonym of Lophiodes insidiator (Regan 1921). Lophiidae. Habitat: marine. Off : carpophaga, Chalceus Valenciennes [A.] in Cuvier & Valenciennes 1850:252 [Histoire naturelle des poissons v. -

La Ricerca Ecologica in Un Mondo Che Cambia
XXVII Congresso Nazionale della Società Italiana di Ecologia La ricerca ecologica in un mondo che cambia Libro degli Abstract 12-15 Settembre 2017 Complesso di SS. Marcellino e Festo, Largo San Marcellino 10, Napoli XXVII Congresso Nazionale della Società Italiana di Ecologia Patrocinato da: XXVII Congresso Nazionale della Società Italiana di Ecologia Sponsorizzato da: XXVII Congresso Nazionale della Società Italiana di Ecologia Comitato scientifico Antonio Mazzola (Università degli Studi di Palermo), Elisa Anna Fano (Università degli Studi di Ferrara), Serena Fonda (Università degli Studi di Trieste), Antonio Pusceddu (Università degli Studi di Cagliari), Salvatrice Vizzini (Università degli Studi di Palermo), Flora Angela Rutigliano (Università degli Studi della Campania “Luigi Vanvitelli”), Caterina Lorenzi (Università degli Studi di Roma “Tor Vergata”), Piero Genovesi (ISPRA), Gianluca Corno (CNR), Gianmarco Giordani (Università degli Studi di Parma). Comitato organizzatore locale Giulia Maisto, Anna De Marco, Carmen Arena, Olga Mangoni, Danilo Russo, Armando Zarrelli (Università degli Studi di Napoli Federico II), Giovanni Russo, Pier Paolo Franzese (Università degli Studi di Napoli Parthenope), Antonietta Fioretto, Flora Angela Rutigliano, Simona Castaldi, Stefania Papa, Giovanna Battipaglia, Rosaria D’Ascoli (Università degli Studi della Campania “Luigi Vanvitelli”), Daniela Baldantoni (Università degli Studi di Salerno), Flavia De Nicola (Università degli Studi del Sannio). Segreteria organizzativa locale Rossana Marzaioli, Valeria -

Rutilus Rutilus Linnaeus, 1758. Rutilo EXÓTICA
Atlas y Libro Rojo de los Peces Continentales de España ESPECIE Rutilus rutilus Linnaeus, 1758. Rutilo EXÓTICA ºo'" o< Q DESCRIPCIÓN Es una especie de talla media que no suele sobrepasar los 40 cm de longitud total aunque se co nocen individuos que han alcanzado los 50 cm de longitud y cerca de los 2 kg de peso. Su cuerpo es alto y comprimido lateralmente, con una cabeza pequeña que representa el 25% de la longitud del cuerpo. La aleta dorsal presenta de 9-11 radios blandos y es alta y de perfil cóncavo. La aleta anal es larga con 9-11 radios blandos. Las escamas son grandes y su número en la línea lateral es de 40-45. Sin dientes mandibulares o maxilares los dientes faríngeos se disponen en una fila en nú mero de 5-5. El número de cromosomas es 2n=50, en algunas poblaciónes es 2n=52. Clase: Actinopterygii Orden: Cypriniformes Familia: Cyprinidae Sinonimias: Cyprinus rutilus Linnaeus, 1758. Leuciscus rutilus (Linnaeus, 1758). Rutilus rutilus (Linnaeus, 1758). Cyprinus ruttilus Linnaeus, 1758. Cyprinus ruhellio Leske, 1774. Cyprinus simus Hermann, 1804. Cyprinus lacustris Pallas, 1814. Cy prinus jaculus ]urine, 1825. Leuciscus decipiens Agassiz, 1835. Leuciscus prasinus Agassiz, 1835. Cyprinus fulvus Vallot, 1837. Cyprinus xanthopterus Vallot, 1837. Rutilus heckelii (Nordmann, 1840). Leuciscus heckelii Nordmann, 1840. Leucos ce nisophius Bonaparte, 1841. Gardonus pigulus Bonaparte, 1841. Leuciscus rutiloides Selys-Longchamps, 1842. Leuciscus sely sii Selys-Longchamps, 1842. Leuciscus lividus Heckel, 1843. Leuciscus pausingeri Heckel, 1843. Leucos pigulus Bonaparte, 1844. Leucos cenisophius Bonaparte, 1845. Leuciscus jurinii Dybowski, 1862. Leuciscus rutilus daugawensis Dybowski, 1862. -

Gardí – Scardinius Erythrophthalmus
Galobart, C., Vila Gispert, A. (2019). Gardí – Scardinius erythrophthalmus. En: Enciclopedia Virtual de los Vertebrados Españoles. López, P., Martín, J., García-Berthou, E. (Eds.). Museo Nacional de Ciencias Naturales, Madrid. http://www.vertebradosibericos.org/ Gardí – Scardinius erythrophthalmus (Linnaeus, 1758) Cristina Galobart y Anna Vila Gispert GRECO, Instituto de Ecología Acuática, Universidad de Girona, 17003 Girona Versión 10-06-2020 ENCICLOPEDIA VIRTUAL DE LOS VERTEBRADOS ESPAÑOLES Sociedad de Amigos del MNCN – MNCN - CSIC Galobart, C., Vila Gispert, A. (2019). Gardí – Scardinius erythrophthalmus. En: Enciclopedia Virtual de los Vertebrados Españoles. López, P., Martín, J., García-Berthou, E. (Eds.). Museo Nacional de Ciencias Naturales, Madrid. http://www.vertebradosibericos.org/ Sinónimos Cyprinus erythrophthalmus Linnaeus, 1758. Leuciscus erythrophthalmus (Linnaeus, 1758). Scardinius erythrophthalmus (Linnaeus, 1758). Scardinius erithrophthalmus (Linnaeus, 1758). Cyprinus erythrops Pallas, 1814. Cyprinus compressus Hollberg, 1822. Cyprinus scardula Nardo, 1827. Scardinius scardafa (Bonaparte, 1832). Cyprinus caeruleus (Yarrell, 1833). Cyprinus fuscus Vallot, 1837. Leuciscus scardafa (Bonaparte, 1837). Scardinius hesperidicus (Bonaparte, 1845). Scardinius platizza Heckel, 1845. Leuciscus apollonitis Richardson, 1857. Scardinius dergle (Heckel y Kner, 1858). Scardinius macrophthalmus Heckel y Kner, 1858. Scardinius plotizza (Heckel y Kner, 1858). Scardinius crocophthalmus Walecki, 1863. Scardinius erythrophthalmus var. dojranensis -

Direct Cytotoxic Activity of CD8+ T Cells Against Ichthyophthirius Multifiliis
426 Abstracts / Fish & Shellfish Immunology 91 (2019) 421e472 importance for stress effects on the immune response in teleosts. Indi- # Corresponding author. vidual aspects of the interference of stress hormones (mainly cortisol) with E-mail address: [email protected] (J. Zou). immune processes have already been reported in some bony fish. Although less studied, the catecholamines adrenaline and noradrenaline have also shown to modulate the immune response of teleost leukocytes via a and b adrenergic receptors. This study aims to expand the actual knowledge on stress-induced immune modulation, in order to evaluate O-014. the effects of stress on the immune system of maraena whitefish (Cor- Direct cytotoxic activity of CD8þ T cells against Ichthyophthirius egonus maraena). This salmonid fish is highly sensitive to stress compared multifiliis in ginbuna crucian carp, Carassius auratus langsdorfii to other salmonid species long adapted to aquaculture. To this end, a large set of specific primers was designed for reverse-transcription quantitative Masaki Sukeda, Koumei Shiota, Takahiro Nagasawa, Miki Nakao, real-time PCR (RT-qPCR) analyses. The primer panel included cell-specific Tomonori Somamoto#. marker genes characterizing the distinct cell populations in the head kidney of C. maraena, which had been sorted using flow cytometry. In Laboratory of Marine Biochemistry, Department of Bioscience and addition, we analysed the expression of catecholamine and cortisol re- Biotechnology, Graduate School of Bioresource and Bioenvironmental ceptors in each population, in order to define the repertoire of stress- Sciences, Kyushu University, Fukuoka, Japan related modulators present in the cells. In the next step, we performed a series of in vitro stimulations of head kidney leukocytes to study the Abstract expression of genes involved in immune activation and acute phase A line of studies has shown that several humoral immune factors including together with catecholamine and cortisol receptors. -

Morfološke Značajke, Taksonomski Položaj I Filogenetičkio Dnosi Populacija Endemskih Vrsta Roda Scardinius (Cypriniformes, Actinopterygii) U Jadranskom Slijevu
Morfološke značajke, taksonomski položaj i filogenetičkio dnosi populacija endemskih vrsta roda Scardinius (Cypriniformes, Actinopterygii) u Jadranskom slijevu Sabolić, Marija Doctoral thesis / Disertacija 2021 Degree Grantor / Ustanova koja je dodijelila akademski / stručni stupanj: University of Zagreb, Faculty of Science / Sveučilište u Zagrebu, Prirodoslovno-matematički fakultet Permanent link / Trajna poveznica: https://urn.nsk.hr/urn:nbn:hr:217:826670 Rights / Prava: In copyright Download date / Datum preuzimanja: 2021-10-06 Repository / Repozitorij: Repository of Faculty of Science - University of Zagreb PRIRODOSLOVNO-MATEMATIČKI FAKULTET BIOLOŠKI ODSJEK Marija Sabolić MORFOLOŠKE ZNAČAJKE, TAKSONOMSKI POLOŽAJ I FILOGENETIČKI ODNOSI POPULACIJA ENDEMSKIH VRSTA RODA SCARDINIUS (CYPRINIFORMES, ACTINOPTERYGII) U JADRANSKOM SLIJEVU DOKTORSKI RAD Zagreb, 2021. FACULTY OF SCIENCE DEPARTMENT OF BIOLOGY Marija Sabolić MORPHOLOGICAL CHARACTERISTICS, TAXONOMIC STATUS AND PHYLOGENETIC RELATIONSHIPS OF THE POPULATIONS OF THE ENDEMIC SCARDINIUS SPECIES (CYPRINIFORMES, ACTINOPTERYGII) IN THE ADRIATIC BASIN DOCTORAL THESIS Zagreb, 2021 „Ovaj je doktorski rad izrađen na Zoologijskom zavodu, pod vodstvom izv. prof. dr. sc. Marka Ćalete, u sklopu Sveučilišnog poslijediplomskog doktorskog studija Biologije pri Biološkom odsjeku Prirodoslovno-matematičkog fakulteta Sveučilišta u Zagrebu“. INFORMACIJE O MENTORU Izv. prof. dr. sc. Ćaleta, Marko Nakon završene I. zagrebačke gimnazije na Biološkom odsjeku PMF-a upisuje studij biologije. Diplomirao je 2000. i stječe zvanje diplomiranog inženjera biologije- ekologije. Poslijediplomski doktorski studij biologije završio je također na Biološkom odsjeku PMF-a, a doktorat znanosti iz znanstvenoga područja prirodnih znanosti, polje biologija, obranio je 2007. godine. Naslov doktorske disertacije glasio je "Ekološke značajke ihtiofaune nizinskog dijela rijeke Save". Od 2000. do 2012. godine zaposlen je u Zoologijskom zavodu Biološkog odsjeka kao mlađi asistent, asistent, viši asistent (poslijedoktorand) i naposljetku kao stručni savjetnik. -

Adding Nuclear Rhodopsin Data Where Mitochondrial COI Indicates Discrepancies – Can This Marker Help to Explain Conflicts in Cyprinids?
DNA Barcodes 2015; 3: 187–199 Research Article Open Access S. Behrens-Chapuis*, F. Herder, H. R. Esmaeili, J. Freyhof, N. A. Hamidan, M. Özuluğ, R. Šanda, M. F. Geiger Adding nuclear rhodopsin data where mitochondrial COI indicates discrepancies – can this marker help to explain conflicts in cyprinids? DOI 10.1515/dna-2015-0020 or haplotype sharing (12 species pairs) with presumed Received February 27, 2015; accepted October 1, 2015 introgression based on mtCOI data. We aimed to test Abstract: DNA barcoding is a fast and reliable tool for the utility of the nuclear rhodopsin marker to uncover species identification, and has been successfully applied reasons for the high similarity and haplotype sharing in to a wide range of freshwater fishes. The limitations these different groups. The included labeonine species reported were mainly attributed to effects of geographic belonging to Crossocheilus, Hemigrammocapoeta, scale, taxon-sampling, incomplete lineage sorting, or Tylognathus and Typhlogarra were found to be nested mitochondrial introgression. However, the metrics for within the genus Garra based on mtCOI. This specific the success of assigning unknown samples to species or taxonomic uncertainty was also addressed by the use genera also depend on a suited taxonomic framework. of the additional nuclear marker. As a measure of the A simultaneous use of the mitochondrial COI and the delineation success we computed barcode gaps, which nuclear RHO gene turned out to be advantageous for were present in 75% of the species based on mtCOI, but the barcode efficiency in a few previous studies. Here, in only 39% based on nuclear rhodopsin sequences.