Polyphyletic Origin of Ornamental Goldfish
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Biogeography and Evolution of the Carassius Auratus-Complex in East
Takada et al. BMC Evolutionary Biology 2010, 10:7 http://www.biomedcentral.com/1471-2148/10/7 RESEARCH ARTICLE Open Access Biogeography and evolution of the Carassius auratus-complex in East Asia Mikumi Takada1,2*, Katsunori Tachihara1, Takeshi Kon2, Gunji Yamamoto2, Kei’ichiro Iguchi3, Masaki Miya4, Mutsumi Nishida2 Abstract Background: Carassius auratus is a primary freshwater fish with bisexual diploid and unisexual gynogenetic triploid lineages. It is distributed widely in Eurasia and is especially common in East Asia. Although several genetic studies have been conducted on C. auratus, they have not provided clear phylogenetic and evolutionary descriptions of this fish, probably due to selection bias in sampling sites and the DNA regions analysed. As the first step in clarifying the evolutionary entity of the world’s Carassius fishes, we attempted to clarify the phylogeny of C. auratus populations distributed in East Asia. Results: We conducted a detailed analysis of a large dataset of mitochondrial gene sequences [CR, 323 bp, 672 sequences (528 sequenced + 144 downloaded); CR + ND4 + ND5 + cyt b, 4669 bp in total, 53 sequences] obtained from C. auratus in East Asia. Our phylogeographic analysis revealed two superlineages, one distributed mainly among the Japanese main islands and the other in various regions in and around the Eurasian continent, including the Ryukyus and Taiwan. The two superlineages include seven lineages with high regional specificity that are composed of endemic populations indigenous to each region. The divergence time of the seven lineages was estimated to be 0.2 million years ago (Mya) by a fossil-based method and 1.0-1.9 Mya by the molecular clock method. -
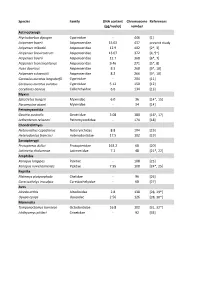
Table S2.Xlsx
Species Family DNA content Chromosome References (pg/nuclei) number Actinopterygii Ptychobarbus dipogon Cyprinidae ‐ 446 [1] Acipenser baerii Acipenseridae 15.02 437 present study Acipenser mikadoi Acipenseridae 12.9 402 [2*, 3] Acipenser brevirostrum Acipenseridae 13.07 372 [4, 5*] Acipenser baerii Acipenseridae 12.7 368 [6*, 7] Acipenser transmontanus Acipenseridae 9.46 271 [5*, 8] Huso dauricus Acipenseridae 8.3 268 [9*, 10] Acipenser schrenckii Acipenseridae 8.2 266 [9*, 10] Carassius auratus langsdorfii Cyprinidae ‐ 204 [11] Carassius auratus auratus Cyprinidae 5.12 150 [12] Corydoras aeneus Callichthyidae 6.6 134 [13] Myxini Eptatretus burgeri Myxinidae 6.0 36 [14*, 15] Paramyxine atami Myxinidae ‐ 34 [14] Petromyzontida Geotria australis Geotriidae 3.08 180 [16*, 17] Lethenteron reissneri Petromyzontidae ‐ 174 [18] Chondrichthyes Notorynchus cepedianus Notorynchidae 8.8 104 [19] Heterodontus francisci Heterodontidae 17.5 102 [19] Sarcopterygii Protopterus dolloi Protopteridae 163.2 68 [20] Latimeria chalumnae Latimeriidae 7.2 48 [21*, 22] Amphibia Xenopus longipes Pipidae ‐ 108 [23] Xenopus ruwenzoriensis Pipidae 7.95 108 [24*, 25] Reptilia Platemys platycephala Chelidae ‐ 96 [26] Carettochelys insculpta Carettochelyidae ‐ 68 [27] Aves Alcedo atthis Alcedinidae 2.8 138 [28, 29*] Upupa epops Upupidae 2.56 126 [28, 30*] Mammalia Tympanoctomys barrerae Octodontidae 16.8 102 [31, 32*] Ichthyomys pittieri Cricetidae ‐ 92 [33] References 1. Yu XY, Yu XJ. A schizothoracin fish species, Diptychus dipogon, with very high number of chromosomes Chrom Inform Serv. 1990;48:17-8. 2. Zhou H, Fujimoto T, Adachi S, Yamaha E, Arai K. Genome size variation estimated by flow cytometry in Acipenser mikadoi, Huso dauricus in relation to other species of Acipenseriformes. -

Electrophoretic Studies of Diploid, Triploid, and Tetraploid Forms of the Japanese Silver Crucian Carp, Carassius Auratus Langsdorfii
Japan.J.Ichthyol. 魚 類 学 雑 誌 40(1):65-75,1993 40(1):65-75,1993 Electrophoretic Studies of Diploid, Triploid, and Tetraploid Forms of the Japanese Silver Crucian Carp, Carassius auratus langsdorfii Yasushi Shimizu,1,2 Takashi Oshiro1 and Mitsuru Sakaizumi2,31 Department of Aquatic Biosciences, Tokyo University of Fisheries, 4-5-7 Konan, Minato-ku, Tokyo 108, Japan 2Department of Experimental Animal Science, The Tokyo Metropolitan Institute of Medical Science, 3-18-22 Honkomagome, Bunkyo-ku, Tokyo 113, Japan 3Present address: Department of Biology, College of General Education, Niigata University, 2-8050 Ikarashi, Niigata 950-21, Japan (Received October 29, 1992; in revisedform February 8, 1993; accepted March 22, 1993) Abstract The diploid-polyploid complex in the Japanese silver crucian carp (ginbuna), Carassius auratus langsdorfii, includes a diploid bisexual form, a triploid gynogenetic form and a tetraploid gynogenetic form. However, the origin of the polyploids remains to be clarified. Flow cytometric and electrophoretic analyses of Japanese crucian carp demonstrated that all the individuals with a two-banded pattern for an AMY-2 gene were polyploid, and 90% of the polyploids gave such a two-banded pattern. One of the two bands was not found in diploid subspecies in Japan but was present in both Korean crucian carp and goldfish. The diploid subspecies, kinbuna (C.auratus subsp.), which is distributed along the Pacific coast of eastern Japan, generated a diagnostic band of phosphoglucomutase. This band was also found in the polyploid "ginbuna" from the same region, but was observed in neither triploids nor diploids from other regions. -

Carps, Minnows Etc. the Cyprinidae Is One of the Largest Fish Families With
SOF text final l/out 12/12/02 12:16 PM Page 60 4.2.2 Family Cyprinidae: Carps, Minnows etc. The Cyprinidae is one of the largest fish families with more than 1700 species world-wide. There are no native cyprinids in Australia. A number of cyprinids have been widely introduced to other parts of the world with four species in four genera which have been introduced to Australia. There are two species found in the ACT and surrounding area, Carp and Goldfish. Common Name: Goldfish Scientific Name: Carassius auratus Linnaeus 1758 Other Common Names: Common Carp, Crucian Carp, Prussian Carp, Other Scientific Names: None Usual wild colour. Photo: N. Armstrong Biology and Habitat Goldfish are usually associated with warm, slow-flowing lowland rivers or lakes. They are often found in association with aquatic vegetation. Goldfish spawn during summer with fish maturing at 100–150 mm length. Eggs are laid amongst aquatic plants and hatch in about one week. The diet includes small crustaceans, aquatic insect larvae, plant material and detritus. Goldfish in the Canberra region are often heavily infected with the parasitic copepod Lernaea sp. A consignment of Goldfish from Japan to Victoria is believed to be responsible for introducing to Australia the disease ‘Goldfish ulcer’, which also affects salmonid species such as trout. Apart from the introduction of this disease, the species is generally regarded as a ‘benign’ introduction to Australia, with little or no adverse impacts documented. 60 Fish in the Upper Murrumbidgee Catchment: A Review of Current Knowledge SOF text final l/out 12/12/02 12:16 PM Page 61 Distribution, Abundance and Evidence of Change Goldfish are native to eastern Asia and were first introduced into Australia in the 1860s when it was imported as an ornamental fish. -

Caring for Your Goldfish
Adding a Goldfish to a Cleaning Your Fish Bowl Dirty fish bowls not only look bad, they Bowl or Aquarium Caring for Now it’s time to put your new Goldfish in are also unhealthy for fish. By following a their new home! Whenever fish are netted few simple maintenance steps your fish Your and handled, their protective slime coat is bowl will always look beautiful. The following steps are an ideal regiment for rubbed off. When adding fish to any keeping your fish bowl looking great. Goldfish aquarium, be sure to add additional water conditioner to help relieve stress. The best To keep your fish healthy, you should method to add new fish is to float the unopened bag of fish in their new home change at least half of the water in your for 10 minutes to allow the fish to adjust Goldfish bowl or aquarium every 3 days. Follow these easy steps: to the water temperature. Then, open the bag and gently release the fish into their 1. Fill a separate container with tap water. Mix hot and cold tap water new home. The bag water may contain fish waste (ammonia), so try to avoid until it is the same temperature as adding the bag water to the aquarium. the water your Goldfish is swimming in. 2. Add a water conditioner to the tap water to remove the disinfectants Feeding Your Fish that are toxic to your fish. It is best to feed your Goldfish only 3. Add the aquarium salts and test the enough food that it can eat in five pH level, adjusting the pH level as minutes. -

AQUATIC INVADERS a Sea Grant/AZA Partnership 1 Goldfish Carassius Auratus • Make Sure That in the Event of a Flood, the Fish, Plants, Snails, Etc
U.S. Geological Survey COMMON NAME: Goldfish SCIENTIFIC NAME: Carassius auratus (Linnaeus 1758) NATIVE DISTRIBUTION: Eastern and central Asia, including China, Russia, Korea, and possibly Japan and Taiwan. U.S. distribution: The goldfish was the first foreign fish introduced into the U. S., and has been here since at least the late 1600s. The species can be found in the wild in every state except Alaska. Habitat: The goldfish is a very adaptable species, and lives in a variety of natural and artificial habitats, Survey Geological U.S. including: lakes, ponds, reservoirs and slow-flowing streams. It is often found in areas with submerged aquatic vegetation. Life cycle: Individuals become adults after about 1 to 2 years. During spawning, the females scatter their adhesive eggs over vegetation, roots, gravel or other sub- strata. The males then fertilize the eggs. Eggs hatch in about 3 to 7 days. Individuals typically live 6 to 7 years. Cool facts: In the wild, the species is a dull olive-brown or bronze. The bright orange goldfish we are familiar with from pet stores is actually a mutation that has been selectively bred for by fish grow- ers. When re-introduced to the wild, reproducing populations of bright orange goldfish quickly revert to the ancestral “wild” type coloration within a few generations. Pathways of invasion: Aquarium releases, bait bucket releases, water garden flooding. Impacts: Goldfish are messy eaters, stirring up the substrate while feeding. This suspension of silt causes excess turbidity in the water, which in turn can kill many kinds of aquatic plants. Additionally, suspended silt can cover the eggs of native species of fish and kill them. -

Leaping Behavior in Silver Carp (Hypophthalmichthys Molitrix): Analysis of Burst Swimming Speeds, Angle of Escape, Height, and Distance of Leaps
CORE Metadata, citation and similar papers at core.ac.uk Provided by eGrove (Univ. of Mississippi) University of Mississippi eGrove Electronic Theses and Dissertations Graduate School 2018 Leaping Behavior In Silver Carp (Hypophthalmichthys Molitrix): Analysis Of Burst Swimming Speeds, Angle Of Escape, Height, And Distance Of Leaps Ehlana Stell University of Mississippi Follow this and additional works at: https://egrove.olemiss.edu/etd Part of the Biology Commons Recommended Citation Stell, Ehlana, "Leaping Behavior In Silver Carp (Hypophthalmichthys Molitrix): Analysis Of Burst Swimming Speeds, Angle Of Escape, Height, And Distance Of Leaps" (2018). Electronic Theses and Dissertations. 374. https://egrove.olemiss.edu/etd/374 This Dissertation is brought to you for free and open access by the Graduate School at eGrove. It has been accepted for inclusion in Electronic Theses and Dissertations by an authorized administrator of eGrove. For more information, please contact [email protected]. LEAPING BEHAVIOR IN SILVER CARP (HYPOPHTHALMICHTHYS MOLITRIX): ANALYSIS OF BURST SWIMMING SPEEDS, ANGLE OF ESCAPE, HEIGHT, AND DISTANCE OF LEAPS A Thesis presented in partial fulfillment of requirements for the degree of Master of Science in the Department of Biology The University of Mississippi By EHLANA G. STELL May 2018 Copyright Ehlana G. Stell ALL RIGHTS RESERVED ABSTRACT Silver Carp have rapidly expanded their range exploiting vulnerable habitats, disrupting fisheries, and inflicting unknown ecological damage. These fish have continued to spread into the Middle Mississippi River and the Tennessee River Valley and great effort is being expended to prevent Silver Carp from entering the Great Lakes and expanding further into the Ohio, Illinois, Missouri, and Tennessee Rivers. -

Moorestown Township Environmental Resource Inventory
APPENDIX C Vertebrate Animals Known or Probable in Moorestown Township Mammals Common Name Scientific Name Status Opossum Didelphis marsupialis Stable Eastern Mole Scalopus aquaticus Stable Big Brown Bat Eptesicus fuscus Stable Little Brown Bat Myotis lucifugus Stable Eastern Cottontail Sylvilagus floridanus Stable Eastern Chipmunk Tamias striatus Stable Gray Squirrel Sciurus carolinensis Stable White-footed Mouse Peromyscus leucopus Stable Meadow Vole Microtus pennsylvanicus Stable Muskrat Ondatra zibethicus Stable Pine Vole Microtus pinetorum Stable Red Fox Vulpes vulpes Stable Gray Fox Urocyon cinereoargenteus Stable Raccoon Procyon lotor Stable Striped Skunk Mephitis mephitis Stable River Otter Lutra canadensis Stable Beaver Castor candensis Increasing White-tailed Deer Odocoileus virginianus Decreasing Source: NJDEP, 2012 C-1 Birds Common Name Scientific Name NJ State Status Loons - Grebes Pied-Billed Grebe Podilymbus podiceps E Gannets - Pelicans - Cormorants Double Crested Cormorant Phalacrocorax auritus S Bitterns - Herons - Ibises American Bittern Botaurus lentiginosus E Least Bittern Ixobrychus exilis SC Black Crowned Night Heron Nycticorax nycticorax T Green Heron Butorides virescens RP Great Blue Heron Ardea herodias SC Great Egret Ardea alba RP Geese - Swans - Ducks Canada Goose Branta canadensis INC Snow Goose Chen caerulescens INC American Wigeon Anas americana S Common Merganser Mergus merganser S Hooded Merganser Lophodytes cucullatus S Green-winged Teal Anas carolinensis RP Mallard Anas platyrhynchos INC Northern Pintail -

Goldfish Vs Betta
Keeping a goldfish in a bowl is not warm for them, and since the recommended for a number of amount of oxygen that can be reasons. dissolved in water decreases as the temperature increases, this The first is that the goldfish you also contributes to their distress. In buy in a store is a baby. It will fact, at Tails we recommend grow over eight inches if given common goldfish for ponds only. the appropriate conditions. The Goldfish story that a fish grows to the size Fancy goldfish are far less hardy of its environment is misleading, that the common ones. They vs. Betta: since it really means that the have been bred over many health of the fish is compromised, centuries to have very round Which is the its growth is stunted, and it dies bodies and large flowing fins. This well before its time. It has been makes them very ornamental, but best first fish as documented that some goldfish not very hardy. They have very have lived for decades – always twisted internal organs and are a pet? in a very large tank, or pond. prone to constipation and swim bladder problems. Heating their By Diane Schickerowsky, B. Sc. The second reason not to put a water a couple of degrees above goldfish in a bowl is that the fish room temperature helps to keep can only breathe air that is things moving through their Fish-keeping is both a fun as well dissolved in the water. A bubbler digestive system and reduces the as educational hobby and fish or filter allows oxygen to be risk of these problems. -

Direct Cytotoxic Activity of CD8+ T Cells Against Ichthyophthirius Multifiliis
426 Abstracts / Fish & Shellfish Immunology 91 (2019) 421e472 importance for stress effects on the immune response in teleosts. Indi- # Corresponding author. vidual aspects of the interference of stress hormones (mainly cortisol) with E-mail address: [email protected] (J. Zou). immune processes have already been reported in some bony fish. Although less studied, the catecholamines adrenaline and noradrenaline have also shown to modulate the immune response of teleost leukocytes via a and b adrenergic receptors. This study aims to expand the actual knowledge on stress-induced immune modulation, in order to evaluate O-014. the effects of stress on the immune system of maraena whitefish (Cor- Direct cytotoxic activity of CD8þ T cells against Ichthyophthirius egonus maraena). This salmonid fish is highly sensitive to stress compared multifiliis in ginbuna crucian carp, Carassius auratus langsdorfii to other salmonid species long adapted to aquaculture. To this end, a large set of specific primers was designed for reverse-transcription quantitative Masaki Sukeda, Koumei Shiota, Takahiro Nagasawa, Miki Nakao, real-time PCR (RT-qPCR) analyses. The primer panel included cell-specific Tomonori Somamoto#. marker genes characterizing the distinct cell populations in the head kidney of C. maraena, which had been sorted using flow cytometry. In Laboratory of Marine Biochemistry, Department of Bioscience and addition, we analysed the expression of catecholamine and cortisol re- Biotechnology, Graduate School of Bioresource and Bioenvironmental ceptors in each population, in order to define the repertoire of stress- Sciences, Kyushu University, Fukuoka, Japan related modulators present in the cells. In the next step, we performed a series of in vitro stimulations of head kidney leukocytes to study the Abstract expression of genes involved in immune activation and acute phase A line of studies has shown that several humoral immune factors including together with catecholamine and cortisol receptors. -

In Vitro Characteristics of Cyprinid Herpesvirus 2: Effect of Kidney Extract Supplementation on Growth
Vol. 115: 223–232, 2015 DISEASES OF AQUATIC ORGANISMS Published August 20 doi: 10.3354/dao02885 Dis Aquat Org In vitro characteristics of cyprinid herpesvirus 2: effect of kidney extract supplementation on growth Tomoya Shibata1, Azusa Nanjo1, Masato Saito1, Keisuke Yoshii1, Takafumi Ito2, Teruyuki Nakanishi3, Takashi Sakamoto1, Motohiko Sano1,* 1Faculty of Marine Science, Tokyo University of Marine Science and Technology, Minato, Tokyo 108-8477, Japan 2Aquatic Animal Health Division, National Research Institute of Aquaculture, Fisheries Research Agency, Tamaki, Mie 519-0423, Japan 3Department of Veterinary Medicine, Nihon University, Fujisawa, Kanagawa 252-0880, Japan ABSTRACT: Herpesviral hematopoietic necrosis caused by goldfish hematopoietic necrosis virus (now identified as cyprinid herpesvirus 2, CyHV-2) has contributed to economic losses in goldfish Carassius auratus culture and is becoming a major obstacle in Prussian carp C. gibelio aquacul- ture in China. Several reports have described difficulties in culturing the virus, with the total loss of infectivity within several passages in cell culture. We succeeded in propagating CyHV-2 with a high infectious titer in a RyuF-2 cell line newly derived from the fin of the Ryukin goldfish variety using culture medium supplemented with 0.2% healthy goldfish kidney extract. The addition of kidney extract to the medium enabled rapid virus growth, resulting in the completion of cyto- pathic effect (CPE) within 4 to 6 d at 25°C. The extract also enabled reproducible virus culture 5−6 −1 with a titer of 10 TCID50 ml . The virus cultured using this protocol showed pathogenicity in goldfish after intraperitoneal injection. The virus grew in RyuF-2 cells at 15, 20, 25, 30, and 32°C but not at 34°C or higher. -

FOSS® at Home Animals Two by Two
FOSS AT HOME FOSS® AT HOME ANIMALS TWO BY TWO ® The FOSS (Full Option Science System™) program offers a number LETTER TO PARENTS of ways to get parents involved in their child’s science education. SCIENCE NEWS Dear Parents, Our kindergarten class is beginning a science unit on animals. W e will be observing and comparing four Included here are short descriptions of several ways to bridge from pairs of animals over the next several weeks: two kinds of fi sh (guppies and goldfi sh), two kinds of snails (land snails and pond snails), two kinds of earthworm (redworms and night crawlers), and two kinds of isopods (pill bugs and sow bugs). W e will learn how to handle these interesting animals carefully and will all participate in the care and feeding of our animal visitors. So be prepared; your child may come classroom to home. home with lots of questions and stories about animals. You can help your child learn about animals by taking walks in your neighborhood to look for animals and by talking about animals in and around your home—everything from pets to insects. W e will be discussing differences and similarities in the animals we investigate and starting to develop the important Letter to Parents. The letter to parents can be sent home at the start attitudes of respect for life and a sense of responsibility where living organisms are concerned. If you are interested in seeing how we introduce animals in our class, please come by for a of a new science module. The letter describes what children will visit.