View PDF Version
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Thesis of Potentially Sweet Dihydrochalcone Glycosides
University of Bath PHD The synthesis of potentially sweet dihydrochalcone glycosides. Noble, Christopher Michael Award date: 1974 Awarding institution: University of Bath Link to publication Alternative formats If you require this document in an alternative format, please contact: [email protected] General rights Copyright and moral rights for the publications made accessible in the public portal are retained by the authors and/or other copyright owners and it is a condition of accessing publications that users recognise and abide by the legal requirements associated with these rights. • Users may download and print one copy of any publication from the public portal for the purpose of private study or research. • You may not further distribute the material or use it for any profit-making activity or commercial gain • You may freely distribute the URL identifying the publication in the public portal ? Take down policy If you believe that this document breaches copyright please contact us providing details, and we will remove access to the work immediately and investigate your claim. Download date: 05. Oct. 2021 THE SYNTHESIS OF POTBTTIALLY SWEET DIHYDROCHALCOITB GLYCOSIDES submitted by CHRISTOPHER MICHAEL NOBLE for the degree of Doctor of Philosophy of the University of Bath. 1974 COPYRIGHT Attention is drawn to the fact that copyright of this thesis rests with its author.This copy of the the sis has been supplied on condition that anyone who con sults it is understood to recognise that its copyright rests with its author and that no quotation from the thesis and no information derived from it may be pub lished without the prior written consent of the author. -

Treatment Protocol Copyright © 2018 Kostoff Et Al
Prevention and reversal of Alzheimer's disease: treatment protocol Copyright © 2018 Kostoff et al PREVENTION AND REVERSAL OF ALZHEIMER'S DISEASE: TREATMENT PROTOCOL by Ronald N. Kostoffa, Alan L. Porterb, Henry. A. Buchtelc (a) Research Affiliate, School of Public Policy, Georgia Institute of Technology, USA (b) Professor Emeritus, School of Public Policy, Georgia Institute of Technology, USA (c) Associate Professor, Department of Psychiatry, University of Michigan, USA KEYWORDS Alzheimer's Disease; Dementia; Text Mining; Literature-Based Discovery; Information Technology; Treatments Prevention and reversal of Alzheimer's disease: treatment protocol Copyright © 2018 Kostoff et al CITATION TO MONOGRAPH Kostoff RN, Porter AL, Buchtel HA. Prevention and reversal of Alzheimer's disease: treatment protocol. Georgia Institute of Technology. 2018. PDF. https://smartech.gatech.edu/handle/1853/59311 COPYRIGHT AND CREATIVE COMMONS LICENSE COPYRIGHT Copyright © 2018 by Ronald N. Kostoff, Alan L. Porter, Henry A. Buchtel Printed in the United States of America; First Printing, 2018 CREATIVE COMMONS LICENSE This work can be copied and redistributed in any medium or format provided that credit is given to the original author. For more details on the CC BY license, see: http://creativecommons.org/licenses/by/4.0/ This work is licensed under a Creative Commons Attribution 4.0 International License<http://creativecommons.org/licenses/by/4.0/>. DISCLAIMERS The views in this monograph are solely those of the authors, and do not represent the views of the Georgia Institute of Technology or the University of Michigan. This monograph is not intended as a substitute for the medical advice of physicians. The reader should regularly consult a physician in matters relating to his/her health and particularly with respect to any symptoms that may require diagnosis or medical attention. -

In Chemistry, Glycosides Are Certain Molecules in Which a Sugar Part Is
GLYCOSIDES Glycosides may be defined as the organic compounds from plants or animal sources, which on enzymatic or acid hydrolysis give one or more sugar moieties along with non- sugar moiety. Glycosides play numerous important roles in living organisms. Many plants store important chemicals in the form of inactive glycosides; if these chemicals are needed, the glycosides are brought in contact with water and an enzyme, and the sugar part is broken off, making the chemical available for use. Many such plant glycosides are used as medications. In animals (including humans), poisons are often bound to sugar molecules in order to remove them from the body. Formally, a glycoside is any molecule in which a sugar group is bonded through its carbon atom to another group via an O-glycosidic bond or an S-glycosidic bond; glycosides involving the latter are also called thioglycosides. The sugar group is then known as the glycone and the non-sugar group as the aglycone or genin part of the glycoside. The glycone can consist of a single sugar group (monosaccharide) or several sugar groups (oligosaccharide). Classification Classification based on linkages Based on the linkage of sugar moiety to aglycone part 1. O-Glycoside:-Here the sugar is combined with alcoholic or phenolic hydroxyl function of aglycone.eg:-digitalis. 2. N-glycosides:-Here nitrogen of amino group is condensed with a sugar ,eg- Nucleoside 3. S-glycoside:-Here sugar is combined with sulphur of aglycone,eg- isothiocyanate glycosides. 4. C-glycosides:-By condensation of a sugar with a cabon atom, eg-Cascaroside, aloin. Glycosides can be classified by the glycone, by the type of glycosidic bond, and by the aglycone. -

Nine Traditional Chinese Herbal Formulas for the Treatment of Depression: an Ethnopharmacology, Phytochemistry, and Pharmacology Review
Journal name: Neuropsychiatric Disease and Treatment Article Designation: Review Year: 2016 Volume: 12 Neuropsychiatric Disease and Treatment Dovepress Running head verso: Feng et al Running head recto: Chinese herbal formulas as antidepressants open access to scientific and medical research DOI: http://dx.doi.org/10.2147/NDT.S114560 Open Access Full Text Article REVIEW Nine traditional Chinese herbal formulas for the treatment of depression: an ethnopharmacology, phytochemistry, and pharmacology review Dan-dan Feng Abstract: Depression is a major mental disorder, and is currently recognized as the Tao Tang second-leading cause of disability worldwide. However, the therapeutic effect of antidepressants Xiang-ping Lin remains unsatisfactory. For centuries, Chinese herbal formulas (CHFs) have been widely used in Zhao-yu Yang the treatment of depression, achieving better therapeutic effects than placebo and having fewer Shu Yang side effects than conventional antidepressants. Here, we review the ethnopharmacology, phy- Zi-an Xia tochemistry, and pharmacology studies of nine common CHFs: “banxia houpo” decoction, “chaihu shugansan”, “ganmaidazao” decoction, “kaixinsan”, “shuganjieyu” capsules, “sinisan”, Yun Wang “wuling” capsules, “xiaoyaosan”, and “yueju”. Eight clinical trials and seven meta-analyses have Piao Zheng supported the theory that CHFs are effective treatments for depression, decreasing Hamilton Yang Wang Depression Scale scores and showing few adverse effects. Evidence from 75 preclinical studies Chun-hu Zhang has also elucidated the multitarget and multipathway mechanisms underlying the antidepres- Laboratory of Ethnopharmacology, sant effect of the nine CHFs. Decoctions, capsules, and pills all showed antidepressant effects, Institute of Integrated Traditional ranked in descending order of efficacy. According to traditional Chinese medicine theory, these Chinese and Western Medicine, Xiangya Hospital, Central South CHFs have flexible compatibility and mainly act by soothing the liver and relieving depression. -
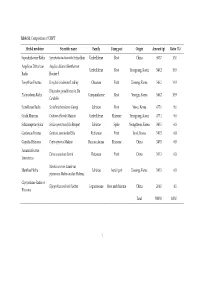
Ratio (%) Saposhnikoviae Ra
Table S1. Composition of CSBPT Herbal medicine Scientific name Family Using part Origin Amount (g) Ratio (%) Saposhnikoviae Radix Saposhnikovia divaricate Schischkin Umbelliferae Root China 500.7 10.0 Angelicae Dahuricae Angelica dahurica Bentham et Umbelliferae Root Yeongyang, Korea 546.2 10.9 Radix Hooker F. Forsythiae Fructus Forsythia viridissima Lindley Oleaceae Fruit Uiseong, Korea 546.2 10.9 Platycodon grandiflorum A. De Platycodonis Radix Campanulaceae Root Yeongju, Korea 546.2 10.9 Candolle Scutellariae Radix Scutellaria baicalensis Georgi Labiatae Root Yeosu, Korea 477.1 9.6 Cnidii Rhizoma Cnidium officinale Makino Umbelliferae Rhizome Yeongyang, Korea 477.1 9.6 Schizonepetae Spica Schizonepeta tenuifolia Briquet Labiatae Spike Yeongcheon, Korea 340.5 6.8 Gardeniae Fructus Gardenia jasminoides Ellis Rubiaceae Fruit Imsil, Korea 340.5 6.8 Coptidis Rhizoma Coptis japonica Makino Ranunculaceae Rhizome China 340.5 6.8 Aurantii Fructus Citrus aurantium Linné Rutaceae Fruit China 340.5 6.8 Immaturus Mentha arvensis Linné var. Menthae Herba Labiatae Aerial part Uiseong, Korea 340.5 6.8 piperascens Malinvaud ex Holmes Glycyrrhizae Radix et Glycyrrhiza uralensis Fischer Leguminosae Root and rhizome China 204.0 4.1 Rhizoma Total 5000.0 100.0 1 Table 2. Chromatographic conditions for simultaneous quantification of compounds 1–18 in CSBPT. Chromatographic parameter Column SunFire C18 analytical column (250 × 4.6 mm, 5 μm) Detector PDA (235, 250, 280, 310, and 345 nm) Flow rate (mL/min) 1.0 Injection volume (μL) 10.0 Column temperature (°C) 40.0 A: 0.1% (v/v) aqueous formic acid Mobile phase B: 0.1% (v/v) formic acid in acetonitrile Time (min) A (%) B (%) 0 95 5 40 40 60 Gradient elution 50 5 95 55 5 95 60 95 5 70 95 5 2 Table S3. -

Simultaneous Determination of Nine Bioactive
Accepted Manuscript Title: Simultaneous determination of nine bioactive compounds in Yijin-tang via high-performance liquid chromatography and liquid chromatography-electrospray ionization-mass spectrometry Author: Hye Jin Yang Nam-Hui Yim Kwang Jin Lee Min Jung Gu Bohyoung Lee Youn-Hwan Hwang Jin Yeul Ma PII: S2213-4220(16)30030-0 DOI: http://dx.doi.org/doi:10.1016/j.imr.2016.04.005 Reference: IMR 200 To appear in: Received date: 14-10-2015 Revised date: 30-3-2016 Accepted date: 7-4-2016 Please cite this article as: Hye Jin YangNam-Hui YimKwang Jin LeeMin Jung GuBohyoung LeeYoun-Hwan HwangJin Yeul Ma Simultaneous determination of nine bioactive compounds in Yijin-tang via high-performance liquid chromatography and liquid chromatography-electrospray ionization-mass spectrometry (2016), http://dx.doi.org/10.1016/j.imr.2016.04.005 This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain. 1 Original Article 2 3 Simultaneous determination of nine bioactive compounds in Yijin-tang via high- 4 performance liquid chromatography and liquid chromatography-electrospray 5 ionization-mass spectrometry 6 7 Hye Jin Yang, Nam-Hui Yim, Kwang Jin Lee, Min Jung Gu, Bohyoung Lee, Youn-Hwan * * 8 Hwang and Jin Yeul Ma 9 10 KM Application Center, Korea Institute of Oriental Medicine, 11 70 Cheomdanro, Dong-gu, Daegu 701-300, Republic of Korea 12 13 Running title: Simultaneous determination of Yijin-tang via HPLC-DAD and LC/MS 14 * 15 Address for co-corresponding author 16 Youn-Hwan Hwang, Ph.D. -

Chemical and Metabolic Profiling of Si-Ni Decoction Analogous Formulae
www.nature.com/scientificreports OPEN Chemical and Metabolic Profiling of Si-Ni Decoction Analogous Formulae by High performance Received: 03 October 2014 Accepted: 26 May 2015 Liquid Chromatography-Mass Published: 29 June 2015 Spectrometry Qian Chen, Shun Xiao, Zhenhao Li, Ni Ai & Xiaohui Fan Along with an indispensable role in healthcare system of China for centuries, Traditional Chinese Medicine (TCM) shows increasing usages as complementary therapy in western countries. To improve our understanding on their therapeutic effects, it’s critical to unveil chemical compositions of TCM formula, the predominant form of therapy in TCM. However, intrinsic chemical complexity makes it a challenging task to perform analysis on each individual TCM formula even with most current state-of- art analytic techniques available. In this work we approached this question by focusing on analogous formulae, a unique category of TCM formulae grouped together based on shared herbs and/or similar TCM syndromes. Systematic chemical profiling on five Si-Ni decoctions (SNs) for cardiovascular diseases was performed by multistage MS and high-resolution MS (HR-MS) experiments. A total of 83 compounds, including alkaloids, flavonoids, ginsenosides, bile acids and triterpenoids, were described. Analysis on SNs-treated rats detected 55 prototype compounds and 39 metabolites in the systemic circulation in vivo, which may contribute directly to their observed clinical efficacies. This approach offers great advantage to speed up identification of chemical compositions of formula and reveal the difference among these analogous formulae that may be related to diverse clinical effects. For centuries Traditional Chinese Medicine (TCM) has been widely used to prevent and treat many common diseases in China and other Asian countries. -

Simultaneous Determination of Nine Bioactive Compounds in Yijin-Tang
integr med res 5 ( 2 0 1 6 ) 140–150 Available online at www.sciencedirect.com Integrative Medicine Research j ournal homepage: www.imr-journal.com Original Article Simultaneous determination of nine bioactive compounds in Yijin-tang via high-performance liquid chromatography and liquid chromatography-electrospray ionization-mass spectrometry Hye Jin Yang, Nam-Hui Yim, Kwang Jin Lee, Min Jung Gu, Bohyoung Lee, ∗ ∗ Youn-Hwan Hwang , Jin Yeul Ma KM Application Center, Korea Institute of Oriental Medicine, Daegu, Korea a r t i c l e i n f o a b s t r a c t Article history: Background: Yijin-tang (YJ) has been used traditionally for the treatment of cardiovascular Received 14 October 2015 conditions, nausea, vomiting, gastroduodenal ulcers, and chronic gastritis. In this study, a Received in revised form simple and sensitive high-performance liquid chromatography (HPLC) method was devel- 30 March 2016 oped for the quantitation of nine bioactive compounds in YJ: homogentisic acid, liquiritin, Accepted 7 April 2016 naringin, hesperidin, neohesperidin, liquiritigenin, glycyrrhizin, 6-gingerol, and pachymic Available online 13 April 2016 acid. Methods: Chromatographic separation of the analytes was achieved on an RS Tech C18 column Keywords: (4.6 mm × 250 mm, 5 m) using a mobile phase composed of water containing 0.1% (v/v) HPLC-DAD trifluoroacetic acid (TFA) and acetonitrile with a gradient elution at a flow rate of 1.0 mL/min. 2 LC/MS Results: Calibration curves for all analytes showed good linearity (R ≥ 0.9995). Lower limits simultaneous determination of detection and lower limits of quantification were in the ranges of 0.03–0.17 g/mL and validation 0.09–0.43 g/mL, respectively. -

Simultaneous Determination of Nine Bioactive Compounds in Yijin-Tang
integr med res 5 ( 2 0 1 6 ) 140–150 Available online at www.sciencedirect.com Integrative Medicine Research j ournal homepage: www.imr-journal.com Original Article Simultaneous determination of nine bioactive compounds in Yijin-tang via high-performance liquid chromatography and liquid chromatography-electrospray ionization-mass spectrometry Hye Jin Yang, Nam-Hui Yim, Kwang Jin Lee, Min Jung Gu, Bohyoung Lee, ∗ ∗ Youn-Hwan Hwang , Jin Yeul Ma KM Application Center, Korea Institute of Oriental Medicine, Daegu, Korea a r t i c l e i n f o a b s t r a c t Article history: Background: Yijin-tang (YJ) has been used traditionally for the treatment of cardiovascular Received 14 October 2015 conditions, nausea, vomiting, gastroduodenal ulcers, and chronic gastritis. In this study, a Received in revised form simple and sensitive high-performance liquid chromatography (HPLC) method was devel- 30 March 2016 oped for the quantitation of nine bioactive compounds in YJ: homogentisic acid, liquiritin, Accepted 7 April 2016 naringin, hesperidin, neohesperidin, liquiritigenin, glycyrrhizin, 6-gingerol, and pachymic Available online 13 April 2016 acid. Methods: Chromatographic separation of the analytes was achieved on an RS Tech C18 column Keywords: (4.6 mm × 250 mm, 5 m) using a mobile phase composed of water containing 0.1% (v/v) HPLC-DAD trifluoroacetic acid (TFA) and acetonitrile with a gradient elution at a flow rate of 1.0 mL/min. 2 LC/MS Results: Calibration curves for all analytes showed good linearity (R ≥ 0.9995). Lower limits simultaneous determination of detection and lower limits of quantification were in the ranges of 0.03–0.17 g/mL and validation 0.09–0.43 g/mL, respectively. -

A Novel Label-Free Fluorescence Assay for Dipeptidyl Peptidase 4
Electronic Supplementary Material (ESI) for ChemComm. This journal is © The Royal Society of Chemistry 2020 Supporting Information A Novel Label-free Fluorescence Assay for Dipeptidyl Peptidase 4 Activity Detection Based on Supramolecular Self-Assembly Yu Zhaoa Jiawen Zoua Yanyan Songb,c Jun Pengd Yi Wang*a Yong Bi*b,c a Pharmaceutical Informatics Institute, College of Pharmaceutical Sciences, Zhejiang University, Hangzhou, 310058, P.R. China. b Department of Neurology, Translational Research Institute of Brain and Brain-Like Intelligence, Shanghai Fourth People's Hospital Affiliated to Tongji University School of Medicine, Shanghai 200081, China. c Department of Cardiology, the First People’s Hospital of Xiaoshan District, Hangzhou, 311200, P. R. China. Corresponding Authors: Yi Wang; [email protected] Yong Bi; [email protected] S-1 Table of contents 1. Experimental section ........................................................................................................3 1.1 Materials ....................................................................................................................3 1.2 Apparatus...................................................................................................................3 1.3 Methods.....................................................................................................................3 2. Results ...............................................................................................................................5 2.1 DPP4 recognition of substrate peptides -

Determination of Flavonoids and Phenolic Acids in Plant Materials Using SLE-SPE-UHPLC-MS/MS Method
Food Analytical Methods https://doi.org/10.1007/s12161-018-1332-9 Determination of Flavonoids and Phenolic Acids in Plant Materials Using SLE-SPE-UHPLC-MS/MS Method Sylwia Bajkacz1 & Irena Baranowska1 & Boguław Buszewski2 & Bartosz Kowalski1 & Magdalena Ligor2 Received: 31 January 2018 /Accepted: 22 July 2018 # The Author(s) 2018 Abstract Today, great emphasis is placed on the search for and dissemination of native plant materials in various industries, with particular emphasis on food, cosmetics, dietary supplements, and as livestock feed and a source of biomass. Solid-liquid extraction (SLE), solid phase extraction (SPE), and ultra-high-performance liquid chromatography coupled with tandem mass spectrometry (UHPLC-MS/MS) methods were developed for the simultaneous determination of 30 flavonoids and phenolic acids in plant materials (lucerne (Medicago sativa L.), goldenrod (Solidago virgaurea L.), phacelia (Phacelia tanacetifolia Benth.), buckwheat (Fagopyrum esculentum), licorice (Glycyrrhiza glabra), and lavender (Lavandula spica L.)). Different SLE methods were tested to evaluate their applicability for the isolation of polyphenols from plants. Then extracts were purified with a C18 reversed-phase SPE cartridge. After extraction, samples were analyzed by UHPLC-MS/MS. The SLE-SPE-UHPLC-MS/MS assay method has been fully validated. For the most compounds, good recoveries and RSDs (< 10%) were obtained. Limits of quantification range from 0.4 to 20 ng/mL. The main phenolic acids in the studied plants have been found to be 3-(4-hydroxyphenyl)propionic acid, 4-hydroxybenzoic acid, and 3,4-dihydroxybenzoic acid. Quercetin, rutin, glabridin, and naringenin are the major flavonoids detected in the analyzed samples. The obtained scientific data can be useful to select plant materials with high nutritional value and the presence of many biologically active ingredients, as well as selecting samples used as a plant biomass, as food, and as a component of dietary supplements. -

Spray Reagents for the Thin Layer Chromatography of Drug Extracts
Spray Reagents For the thin layer chromatography of drug extracts No. 1 Anisaldehyde-acetic acid reagent (AA) 0.5 ml Anisaldehyde mixed with 10 ml of 98% acetic acid. The TLC plate is sprayed with 5-10 ml, then heated at 120 0 C for 7-10 min in a drying cabinet. Detection 0/ petasin/isopetasin. The plate may be sprayed afterwards with conc. sulphuric acid and evaluated in vis. or UV-365 nm. NO.2 Anisaldehyde-sulphuric acid reagent (AS) 0.5 ml Anisaldehyde is mixed with 10 ml glacial acetic acid, followed by 85 ml metha nol and 5 ml concetrated sulphuric acid, in that order. The re agent has only limited stability, and is no longer useable when the colour has turned to red-violet. The TLC plate is sprayed with ab out 10 ml, heated at 100° C for 5-10 min, then evaluated in vis. or UV-365 nm. Detection 0/ essential oils, pungent principles, bitter principles, saponins, etc. NO.3 Antimony(lII) chloride reagent (SbCI 3) 20% solution of antimony(III) chloride in chloroform. The TLC plate must be sprayed with 15-20 ml reagent, then heated for 5-6 min at 100° C. Evaluation in vis. or UV-365 nm. Detection 0/ cardiac glycosides, saponins, visnagin (Ammi visnagae /ructus), etc. NO.4 Benzidine re agent (BZ) 0.5 g Benzidine is dissolved in 10 ml glacial acetic acid and the volume adjusted to 100 ml with ethanol. Evaluation in vis. Detection 0/ aucubin (Plantaginis folium) NO.5 Berlin blue reagent (BB) A freshly prepared solution of 10 g iron(III) chloride and 0.5 g potassium hexacyano ferrate in 100 ml water.