Satellite Article Cerebrospinal Fluid Collection and Its Analysis in Equine Neurological Disease B
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Cerebrospinal Fluid in Critical Illness
Cerebrospinal Fluid in Critical Illness B. VENKATESH, P. SCOTT, M. ZIEGENFUSS Intensive Care Facility, Division of Anaesthesiology and Intensive Care, Royal Brisbane Hospital, Brisbane, QUEENSLAND ABSTRACT Objective: To detail the physiology, pathophysiology and recent advances in diagnostic analysis of cerebrospinal fluid (CSF) in critical illness, and briefly review the pharmacokinetics and pharmaco- dynamics of drugs in the CSF when administered by the intravenous and intrathecal route. Data Sources: A review of articles published in peer reviewed journals from 1966 to 1999 and identified through a MEDLINE search on the cerebrospinal fluid. Summary of review: The examination of the CSF has become an integral part of the assessment of the critically ill neurological or neurosurgical patient. Its greatest value lies in the evaluation of meningitis. Recent publications describe the availability of new laboratory tests on the CSF in addition to the conventional cell count, protein sugar and microbiology studies. Whilst these additional tests have improved our understanding of the pathophysiology of the critically ill neurological/neurosurgical patient, they have a limited role in providing diagnostic or prognostic information. The literature pertaining to the use of these tests is reviewed together with a description of the alterations in CSF in critical illness. The pharmacokinetics and pharmacodynamics of drugs in the CSF, when administered by the intravenous and the intrathecal route, are also reviewed. Conclusions: The diagnostic utility of CSF investigation in critical illness is currently limited to the diagnosis of an infectious process. Studies that have demonstrated some usefulness of CSF analysis in predicting outcome in critical illness have not been able to show their superiority to conventional clinical examination. -

Spontaneous Spinal Cerebrospinal Fluid Leaks and Intracranial Hypotension
CLINICAL REVIEW CLINICIAN’S CORNER Spontaneous Spinal Cerebrospinal Fluid Leaks and Intracranial Hypotension Wouter I. Schievink, MD Context Spontaneous intracranial hypotension is caused by spontaneous spinal ce- PATIENT PRESENTS WITH A rebrospinal fluid (CSF) leaks and is known for causing orthostatic headaches. It is an new headache that occurs important cause of new headaches in young and middle-aged individuals, but initial shortly after assuming an up- misdiagnosis is common. right position and is re- Objective To summarize existing evidence regarding the epidemiology, pathophysi- Alieved by lying down. Although such a ology, diagnosis, and management of spontaneous spinal CSF leaks and intracranial positional headache pattern is well- hypotension. known following a diagnostic lumbar Evidence Acquisition MEDLINE (1966-2005) and OLDMEDLINE (1950-1965) were puncture, the spontaneous onset of an searched using the terms intracranial hypotension, CSF leak, low pressure headache, orthostatic headache is not well recog- and CSF hypovolemia. Reference lists of these articles and ongoing investigations in nized and the patient may be diag- this area were used as well. nosed with migraine, tension head- Evidence Synthesis Spontaneous intracranial hypotension is caused by single or ache, viral meningitis, or malingering. multiple spinal CSF leaks. The incidence has been estimated at 5 per 100 000 per year, This has been a typical scenario for with a peak around age 40 years. Women are affected more commonly than men. Mechanical factors combine with an underlying connective tissue disorder to cause many patients experiencing spontane- 1 the CSF leaks. An orthostatic headache is the prototypical manifestation but other head- ous intracranial hypotension. -

Pseudomigraine with Pleocytosis
P1: KWW/KKL P2: KWW/HCN QC: KWW/FLX T1: KWW GRBT050-115 Olesen- 2057G GRBT050-Olesen-v6.cls August 17, 2005 1:50 ••Chapter 115 ◗ Pseudomigraine with Pleocytosis Dimos D. Mitsikostas and Julio Pascual Other terms: migraine variants, headache and neurologic dle cerebral artery (9). Alternatively, imaging studies sug- deficits with cerebrospinal fluid lymphocytosis (HaNDL) gest that neurologic deficits of HaDNL might be caused by cortical spreading depression (5,6), as in migraine with aura. Furthermore, overlap of clinical presentations be- INTRODUCTION tween HaNDL and familial hemiplegic migraine triggered a search for mutations in the CACNA1A gene, which were Pseudomigraine is a term first introduced in 1983 to not found (4). Finally, the CSF inflammatory picture of describe attacks of temporary neurologic deficit accom- HaNDL suggests an inflammatory, autoimmune, or postin- panied or followed by migrainous headache and cere- fectious etiology, and supportive data are awaited. brospinal fluid (CSF) pleocytosis of unknown origin and automatic relief (10). Similar clinical presentations were reported previously under different names, such as mi- PRESENTATION AND EVALUATION graine variants (1,13). Diagnostic criteria (Revised International Classification for Headache Disorders [ICHD-II]) of HaNDL are listed EPIDEMIOLOGY in Table 115-1. The clinical characteristics of HaNDL are: (1) more than two discrete episodes of temporal focal Fewer than 100 cases of HaNDL are reported in the liter- neurologic (most often sensory plus aphasic) symptoms ature (2,7), but it may be underdiagnosed as estimates of followed by moderate to severe headache and (2) com- its annual incidence are up to 0.2 cases per 100,000 inhab- plete and spontaneous relief of the condition after a few itants (11). -

CSF Xanthochromia
CSF Xanthochromia Pseudonyms – CSF bilirubin For investigation of suspected subarachnoid haemorrhage (SAH) in CT negative patients Xanthochromia is the yellow discoloration indicating the presence of bilirubin in CSF which appears as oxyhaemoglobin released from the breakdown of red blood cells following haemorrhage into the CSF is converted in vivo into bilirubin in a time‐dependent manner. A subarachnoid haemorrhage (SAH) is a spontaneous arterial bleeding into the subarachnoid space, usually from a cerebral aneurysm, and characterised by a severe sudden‐onset headache. The majority of positive cases are detected by computed tomography (CT) scanning but for those CT‐ negative patients presenting with a history suggestive of SAH the measurement of xanthochromia in CSF is advocated to detect those patients who actually have sustained a SAH and require treatment and to eliminate the possibility of SAH in the remainder without the need of confirmatory angiography. The CSF is collected by means of lumbar puncture (LP). For full interpretation of the result other CSF and blood tests must be collected at the same time – CSF protein and glucose; plasma glucose; and serum protein and bilirubin. General information CSF Xanthochromia sample collection kits are provided in the ACU (ORC) and AMU (Trafford) and also available from the Specimen Receptions at the laboratories at both sites. Concurrent samples should be requested for CSF protein and glucose, plasma glucose and serum LFTS (for protein and bilirubin) using the sample containers supplied. Collection container: CSF xanthochromia: White topped Universal container (In addition the following samples should be collected:‐ CSF glucose & protein: 1.2 mL fluoride‐EDTA glucose (Sarstedt yellow top) Serum protein & bilirubin: 4.9 mL SST (Sarstedt brown top) Plasma glucose: 2.7 mL fluoride‐EDTA glucose (Sarstedt yellow top) Type and volume of sample: 1 mL CSF requested, minimum 400 µL required for analysis Specimen transport/special precautions: A CSF Xanthochromia sample collection kit should be used. -
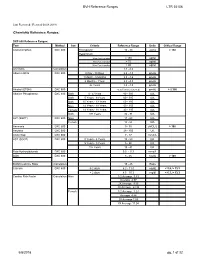
BVH Reference Ranges LTR 35106 Chemistry Reference Ranges
BVH Reference Ranges LTR 35106 Last Reviewed: (Revised 05.08.2018) Chemistry Reference Ranges: DXC 600 Reference Ranges: Test Method Sex Criteria Reference Range Units Critical Range Acetaminophen DXC 600 Therapeutic 10 - 30 ug/mL > 150 Hepatotoxic 4 hrs Post Ingestion > 150 ug/ml 8 hrs Post Ingestion > 75 ug/ml 12 hrs Post Ingestion > 40 ug/ml A/G Ratio Calculation 1.1 - 2.2 Albumin BCG DXC 600 0 Day - 30 Days 2.6 - 4.3 gm/dL 1 Month - 5 Months 2.8 - 4.6 gm/dL 6 Months - 1Year 2.8 - 4.8 gm/dL 2+ Years 3.2 - 4.9 gm/dL Alcohol (ETOH) DXC 600 <0.005 (none detected) gm/dL > 0.300 Alkaline Phosphatase DXC 600 Both 0 - 4 Years 80 - 350 IU/L Both 5 Years - 9 Years 60 - 385 IU/L Both 10 Years - 13 Years 60 - 485 IU/L Male 14 Years - 18 Years 50 - 350 IU/L Female 14 Years - 18 Years 40 - 195 IU/L Both 19+ Years 32 - 91 IU/L ALT (SGPT) DXC 600 Male 17 - 63 IU/L Female 14 - 54 IU/L Ammonia DXC 600 9 - 35 uMOL/L > 100 Amylase DXC 600 28 - 100 U/L Anion Gap DXC 600 7 - 17 mmol/L AST (SGOT) DXC 600 0 Years - 4 Years 10 - 60 IU/L 5 Years - 9 Years 5 - 50 IU/L 10+ Years 15 - 41 IU/L Beta Hydroxybutyrate DXC 600 0.0 - 0.3 mmol/L BUN DXC 600 8 - 26 mg/dL > 100 BUN/Creatinine Ratio Calculation 15 - 25 Ratio Calcium DXC 600 0-2 days 6.2 - 11.0 mg/dL < 5.8, > 13.3 > 2 days 8.5 - 10.3 mg/dl < 6.3, > 13.3 Cardiac Risk Factor Calculation Male 1/2 Average 3.43 Average 4.97 2X Average 9.55 3X Average 23.99 Female 1/2 Average 3.27 Average 4.44 2X Average 7.05 3X Average 11.04 5/8/2018 pg. -

Routine Cerebrospinal Fluid (CSF) Analysis
CHAPTER4 Routine cerebrospinal fluid (CSF) analysis a b a ) F. Deisenhammer, A. Bartos, R. Egg, Albumin CSF/serum ratio (Qalb should be pre- N. E. Gilhus,c G. Giovannoni,d S. Rauer,e ferred to total protein measurement and normal F. Sellebjergf upper limits should be related to patients’ age. Elevated Qalb is a non-specific finding but occurs mainly in bacterial, cryptococcal, and tubercu- Background A great variety of neurological diseases lous meningitis, leptomingeal metastases as well require investigation of the cerebrospinal fluid as acute and chronic demyelinating polyneu- (CSF) to prove the diagnosis or to rule out relevant ropathies. differential diagnoses. Pathological decrease of the CSF/serum glu- cose ratio or an increase in lactate concentration Objectives To evaluate the theoretical background indicates bacterial or fungal meningitis or lep- and provide guidelines for clinical use in rou- tomeningeal metastases. tine CSF analysis including total protein, albumin, Intrathecal immunoglobulin G synthesis is best immunoglobulins, glucose, lactate, cell count, demonstrated by isoelectric focusing followed by cytological staining, and investigation of infec- specific staining. tious CSF. Cellular morphology (cytological staining) should be evaluated whenever pleocytosis is found or Methods Systematic Medline search for the above leptomeningeal metastases or pathological bleed- mentioned variables. Review of appropriate publi- ing is suspected. Computed tomography-negative cations by one or more of the task force members. intrathecal bleeding should be investigated by Grading of evidence and recommendations was bilirubin detection. based on consensus by all task force members. Introduction CSF should be analysed immediately after collec- tion. If storage is needed 12 ml of CSF should The cerebrospinal fluid (CSF) is a dynamic, be partitioned into three to four sterile tubes. -
D40. Cerebrospinal Fluid.Pdf
CEREBROSPINAL FLUID D40 (1) Cerebrospinal Fluid (CSF) Last updated: June 3, 2019 PHYSIOLOGY ............................................................................................................................................ 1 CSF PRODUCTION .................................................................................................................................. 1 CSF REABSORPTION ............................................................................................................................... 1 PARAMETERS ........................................................................................................................................... 2 NORMAL ................................................................................................................................................ 2 OPENING PRESSURE ................................................................................................................................ 3 COLOR ................................................................................................................................................... 3 BLOODY CSF ......................................................................................................................................... 3 VISCOSITY & TURBIDITY ....................................................................................................................... 4 CELLS ................................................................................................................................................... -

2017 LAB GUIDE TEST MENU March 2017Edition
2017 LAB GUIDE TEST MENU March 2017Edition Replaces: Sept 2015 Edition STAT TESTING MENU (2 Pages) 30 Minute In-Lab Turnaround Time (TAT) Unless Further Noted Below (In-Lab Time) MICROBIOLOGY 1. Spinal Fluid Culture set up and Gram Stain of sediment, India Ink prep 2. Gram Stain, other sites 3. Planting of cultures 4. Wet prep 5. Rapid Strep A Antigen Test (15 min. TAT) 6. Rapid Influenza A & B Antigen test 7. Rapid RSV Antigen test 8. Malaria Prep (60 min. TAT), Evening and Night shifts will only report “Parasites present or absent” 9. Screen, Gastric Urease, for Helicobacter Pylori (60 min. TAT) 10. Clostridium difficile DNA amplification (60 min. TAT) BLOOD BANK 1. Compatibility testing (leuko-reduced packed cells) 2. Type and Screen (60 min. TAT) 3. Cord Blood (2 hours) HEMATOLOGY 1. Complete Blood Count (CBC) SEE NOTE #1 2. Fibrinogen 3. Hemogram (ABC) 4. Monospot Test 5. Partial Thromboplastin Time (PTT) (60 min. TAT) 6. Prothrombin Time (PT) (60 min. TAT) 7. Fluid Cell Count (60 min. TAT) 8. Complete Urinalysis 9. D-dimer 10. Fluid pH CHEMISTRY 1. Arterial Blood Gases 2. Acetone 3. Ammonia 4. Amylase 5. Basic Metabolic Panel: Sodium, Potassium, Chloride, CO2, Creatinine, BUN, Glucose, and Calcium 6. Bilirubin (Total) 7. Blood Urea Nitrogen (BUN) 8. Calcium (Total and Ionized) 9. CPK (Total) (60 min. TAT for CPK total with MB fraction. CPKMB only run if CPK total > 113) UVM HEALTH NETWORK-CVPH 2 TEST MENU 10. Creatinine 11. CSF (Glucose and Protein) 12. Electrolytes: Sodium (Na), Potassium (K), Chloride (Cl), CO2 13. -

Effects of Hyperglycemia and Rapid Lowering of Plasma Glucose in Normal Rabbits
MILESTONES IN NEPHROLOGY J Am Soc Nephrol 11: 1776–1788, 2000 Mark A. Knepper, Feature Editor Studies on Mechanisms of Cerebral Edema in Diabetic Comas EFFECTS OF HYPERGLYCEMIA AND RAPID LOWERING OF PLASMA GLUCOSE IN NORMAL RABBITS ALLEN I. ARIEFF AND CHARLES R. KLEEMAN WITH THE TECHNICAL ASSISTANCE OF ALICE KEUSHKERIAN AND HELEN BAGDOYAN From the Departments of Medicine, Wadsworth Veterans Administration Center and Cedars-Sinai Medical Center, and the Cedars-Sinai Medical Research Institute, and University of California Los Angeles Medical Center, Los Angeles, California 90048 with comments by ALLEN I. ARIEFF AND RICHARD STERNS Reprinted from J. Clin. Invest. 52:571–583, 1973 A BSTRACT To investigate the pathophysiology of cerebral edema occurring during treatment of diabetic coma, the effects of hyperglycemia and rapid lowering of plasma glucose were AUTHOR COMMENTARY evaluated in normal rabbits. During 2 h of hyperglycemia (plasma glucose = 61 mM), both brain (cerebral cortex) and Allen I. Arieff muscle initially lost about 10% of water content. After 4 h of hyperglycemia, skeletal muscle water content remained low University of California, but that of brain was normal. Brain osmolality (Osm) (343 San Francisco mosmol/kg H2O) was similar to that of cerebrospinal fluid Sausalito, California (CSF) (340 mosmol/kg), but increases in the concentration of Na+, K+, Cl–, glucose, sorbitol, lactate, urea, myoinositol, and amino acids accounted for only about half of this increase. n 1936, Dillon, Riggs, and Dyer described a syndrome The unidentified solute was designated “idiogenic osmoles”. Iwhereby individuals who were being treated for diabetic When plasma glucose was rapidly lowered to normal with coma and apparently recovering suddenly deteriorated, with insulin, there was gross brain edema, increases in brain content worsening of coma, respiratory insufficiency, hypotension, of water, Na+, K+, Cl– and idiogenic osmoles, and a signifi tachycardia, high fever, and death (1). -

Surgical Treatment of Spontaneous Spinal Cerebrospinal Fluid Leaks
Neurosurg Focus 9 (1):Article 7, 2000, Click here to return to Table of Contents Surgical treatment of spontaneous spinal cerebrospinal fluid leaks CORMAC O. MAHER, M.D., FREDRIC B. MEYER, M.D., AND BAHRAM MOKRI, M.D. Departments of Neurosurgery and Neurology, Mayo Clinic, Rochester, Minnesota Spontaneous spinal cerebrospinal fluid (CSF) leaks are an increasingly recognized cause of intracranial hypoten- sion. In this report the authors review the indications for surgery, surgical techniques, and surgery-related outcomes for these lesions. The major presenting symptoms include postural headaches, nausea, vomiting, and diplopia. Often, there is no history of traumatic injury. The most common cranial magnetic resonance (MR) imaging features include pachymeningeal gadolinium enhancement and sagging of the brain. On spinal MR images, diverticula are frequently noted. In cases in which symptoms are severe and refractory to less invasive measures, surgical intervention is indi- cated. Tears in the dura or leaking diverticula that are identified as the sources of the CSF leak often can be ligated or repaired. When a source of CSF egress is not found intraoperatively, packing the epidural space with blood-soaked Gelfoam or muscle at the appropriate level can lead to relief of symptoms. Occasionally the dural defect is large, irreg- ular, or has attenuated borders that may not be possible to repair with sutures. These may be repaired by packing the defect with muscle or blood-soaked Gelfoam. Indications for and outcomes of surgery in patients with this condition will become more defined as surgeons gain experience with these procedures. KEY WORDS • cerebrospinal fluid leak • intracranial hypotension • intracranial pressure The syndrome of low-pressure CSF headaches was bar puncture. -

A Case Report on Syndrome of Transient Headache and Neurological Deficits
A CASE REPORT ON SYNDROME OF TRANSIENT HEADACHE AND NEUROLOGICAL DEFICITS WITH CEREBROSPINAL FLUID LYMPHOCYTOSIS (HANDL) MAHMUD R1, RASSEL MA2, MONAYEM FB3, CHOWDHURY AH4, ISLAM KMN5 Abstract: HaNDL syndrome (transient headache and neurological deficits with cerebrospinal fluid lymphocytosis) is a rare headache disorder previously known as Migraine with cerebrospinal fluid pleocytosis. The disease is characterized by one or more episodes of headache and transient neurological deficits associated with cerebrospinal fluid lymphocytosis. It is a benign disorder and a stroke mimicker. We describe a case of 50 years old lady presented with acute onset headache, right hemi sensory tingling and bilateral papilloedema. Her blood test, MRI of brain and MRV was normal. CSF study revealed lymphocytic pleocytosis. The patient was discharged with full recovery. HaNDL syndrome is a diagnosis of exclusion. High degree of suspicion and characteristic clinical and laboratory findings are important to recognize. Key word: HaNDL, Pleocytosis, Migraine DOI: https://doi.org/10.3329/jdmc.v29i1.51177 J Dhaka Med Coll. 2020; 29(1) : 89-91 Introduction: Case Description: The syndrome of transient headache and A 50 year old lady admitted in the inpatient of neurologic deficits with cerebrospinal fluid the Neurology Department of Dhaka Medical lymphocytosis (HaNDL syndrome) is a self- College on 15.02.20 with the history of severe limited condition. It was previously known as throbbing head ache for 2 days associated with Migraine with cerebrospinal pleocytosis; pseudo tingling sensation on the he right side of her migraine with lymphocytic pleocytosis. It was body. The headache was global, throbbing in first described in early 19801. -

Tests…But Maybe You Transports Don’T 2
11/12/15 Some things you should When lab tests are useful know about laboratory 1. Managing patients during critical care tests…But maybe you transports don’t 2. While transporting patient to medical facilities for evaluation of laboratory Steve Faynor, CCEMT-P abnormalities HCA Chippenham Medical Center Richmond Ambulance Authority Objectives 1. Review some basic laboratory tests. Treat the patient, not the 2. Appreciate how patterns of laboratory test results can offer insight into etiology. laboratory values. 3. Learn how laboratory test calculations can add additional clinical information. 4. Review some limitations of laboratory tests. ELECTROLYTES & A case of “bad labs” RENAL FUNCTION TESTS 1 11/12/15 Hypernatremia & Renal Failure Hypernatremia • 89 year old white female • Hyperaldosteronism • Coming from nursing home due to • Cushing’s disease or syndrome abnormal labs • Diabetes insipidus (deficiency of ADH) • Sodium 172 mmol/L • Dehydration • Potassium 4.2 mmol/L • Chloride 137 mmol/L • Carbon dioxide 21 mmol/L • What are some causes of hypernatremia? • BP 122/66, SBP 99 later • BUN 212 mg N/dL • HR 64/min • Creatinine 6.10 mg/dL • RR 21/min • What do these values indicate? • SpCO2 98% on 4 L oxygen per min • Does this change your therapy? • Tongue dry, skin turgor poor • What is the cause of the hypernatremia in this patient? Treatment? Acute Renal Failure Use of the BUN/creatinine ratio • Intrinsic renal disease • In intrinsic causes of acute renal failure, the – Acute tubular necrosis: ischemia, toxins BUN/creatinine ratio is typically 10-15. – Acute glomerulonephritis • In pre-renal causes of acute renal failure, – CKD with missed dialysis the BUN/creatinine ratio is typically >20.