2017 LAB GUIDE TEST MENU March 2017Edition
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Hba1c %, Total Serum Protein and Albumin Levels in Type 2 Diabetes Mellitus Patients: a Case-Control Study Dr
European Journal of Molecular & Clinical Medicine (EJMCM) ISSN: 2515-8260 Volume 07, Issue 11, 2020 Original research article HbA1c %, total serum protein and albumin levels in type 2 diabetes mellitus patients: a case-control study Dr. Shweta Kumari1*, Dr. Supriya2 1Junior Resident (Academic), Department of Pathology Darbhanga Medical College and Hospital, Laheriasarai, Darbhanga, Bihar, India 2Junior Resident (Academic), Department of Pathology Darbhanga Medical College and Hospital, Laheriasarai, Darbhanga, Bihar, India Corresponding Author: Dr. Shweta Kumari Abstract Aim: the aim of the study to assessment of glycated haemoglobin, total protein and albumin levels in patients with type 2 diabetes mellitus. Methods: This case control study was done the Department of Pathology Darbhanga Medical College and Hospital, Laheriasarai, Darbhanga, Bihar, India, for 1 year. The research enlisted 100 participants, 50 of whom were diabetic and 50 of whom were not, all of whom were between the ages of 40 and 70. Every patient's blood sample was obtained in 5mls, with 1ml dispensed into EDTA for glycated haemoglobin estimation and 4ml dispensed into clear containers for serum albumin and total protein estimation. Normal procedures were used to determine the amount of glycated haemoglobin, estimate serum albumin, and estimate total protein. Results: The mean level of HbA1c was significantly higher in the diabetic subjects when compared with control group (10.11±1.41Vs 6.18±0.71; p=0.000). There was no significant differences observed between the age, the serum levels of Albumin and Total protein in the test and control subjects (p>0.05). Conclusion: The present study showed significantly higher mean levels of HbA1c in the diabetic patients compared with the control subjects. -

Cerebrospinal Fluid in Critical Illness
Cerebrospinal Fluid in Critical Illness B. VENKATESH, P. SCOTT, M. ZIEGENFUSS Intensive Care Facility, Division of Anaesthesiology and Intensive Care, Royal Brisbane Hospital, Brisbane, QUEENSLAND ABSTRACT Objective: To detail the physiology, pathophysiology and recent advances in diagnostic analysis of cerebrospinal fluid (CSF) in critical illness, and briefly review the pharmacokinetics and pharmaco- dynamics of drugs in the CSF when administered by the intravenous and intrathecal route. Data Sources: A review of articles published in peer reviewed journals from 1966 to 1999 and identified through a MEDLINE search on the cerebrospinal fluid. Summary of review: The examination of the CSF has become an integral part of the assessment of the critically ill neurological or neurosurgical patient. Its greatest value lies in the evaluation of meningitis. Recent publications describe the availability of new laboratory tests on the CSF in addition to the conventional cell count, protein sugar and microbiology studies. Whilst these additional tests have improved our understanding of the pathophysiology of the critically ill neurological/neurosurgical patient, they have a limited role in providing diagnostic or prognostic information. The literature pertaining to the use of these tests is reviewed together with a description of the alterations in CSF in critical illness. The pharmacokinetics and pharmacodynamics of drugs in the CSF, when administered by the intravenous and the intrathecal route, are also reviewed. Conclusions: The diagnostic utility of CSF investigation in critical illness is currently limited to the diagnosis of an infectious process. Studies that have demonstrated some usefulness of CSF analysis in predicting outcome in critical illness have not been able to show their superiority to conventional clinical examination. -

International Journal of Medical and Biomedical Studies (IJMBS)
|| ISSN(online): 2589-8698 || ISSN(print): 2589-868X || International Journal of Medical and Biomedical Studies Available Online at www.ijmbs.info PubMed (National Library of Medicine ID: 101738825) Index Copernicus Value 2018: 75.71 Original Research Article Volume 4, Issue 3; March: 2020; Page No. 209-213 EVALUATION OF CHANGES IN LEVELS OF GLYCATED HEMOGLOBIN, TOTAL PROTEIN AND ALBUMIN IN PATIENTS DIAGNOSED WITH TYPE 2 DIABETES MELLITUS Dr. Vivek Kumar1, Dr. Neeraj Kumar2, Dr. Jaideo Prasad3 1Tutor, Dept. of Pathology, Anugrah Narayan Magadh Medical College and Hospital, Gaya, Bihar, India. 2Senior Resident, Dept. of Medicine, Anugrah Narayan Magadh Medical College and Hospital, Gaya, Bihar, India. 3Prof & HOD, Dept. of Pathology, Anugrah Narayan Magadh Medical College and Hospital, Gaya, Bihar, India. Article Info: Received 07 February 2020; Accepted 26 March 2020 DOI: https://doi.org/10.32553/ijmbs.v4i3.1099 Corresponding author: Dr. Neeraj Kumar Conflict of interest: No conflict of interest. Abstract Insulin resistance in Type 2 diabetes mellitus metabolism of carbohydrates, lipids and proteins gives an estimate of the average blood glucose the previous three months in diabetes. Protein and HbA1c have been shown to be involved complications of diabetes mellitus. Hence based on above findings the present study was planned for Evaluation of Changes in Levels of Glycated Hemoglobin, Total Protein and Albumin in Patients Diagnosed with Type 2 Diabetes Mellitus. The present study was planned in Anugrah Narayan Magadh Medical College and Hospital, Gaya, Bihar, India. In the present study 50 cases were evaluated. The 25 cases were enrolled on the Group A as normal cases in Control group. -

CSF Xanthochromia
CSF Xanthochromia Pseudonyms – CSF bilirubin For investigation of suspected subarachnoid haemorrhage (SAH) in CT negative patients Xanthochromia is the yellow discoloration indicating the presence of bilirubin in CSF which appears as oxyhaemoglobin released from the breakdown of red blood cells following haemorrhage into the CSF is converted in vivo into bilirubin in a time‐dependent manner. A subarachnoid haemorrhage (SAH) is a spontaneous arterial bleeding into the subarachnoid space, usually from a cerebral aneurysm, and characterised by a severe sudden‐onset headache. The majority of positive cases are detected by computed tomography (CT) scanning but for those CT‐ negative patients presenting with a history suggestive of SAH the measurement of xanthochromia in CSF is advocated to detect those patients who actually have sustained a SAH and require treatment and to eliminate the possibility of SAH in the remainder without the need of confirmatory angiography. The CSF is collected by means of lumbar puncture (LP). For full interpretation of the result other CSF and blood tests must be collected at the same time – CSF protein and glucose; plasma glucose; and serum protein and bilirubin. General information CSF Xanthochromia sample collection kits are provided in the ACU (ORC) and AMU (Trafford) and also available from the Specimen Receptions at the laboratories at both sites. Concurrent samples should be requested for CSF protein and glucose, plasma glucose and serum LFTS (for protein and bilirubin) using the sample containers supplied. Collection container: CSF xanthochromia: White topped Universal container (In addition the following samples should be collected:‐ CSF glucose & protein: 1.2 mL fluoride‐EDTA glucose (Sarstedt yellow top) Serum protein & bilirubin: 4.9 mL SST (Sarstedt brown top) Plasma glucose: 2.7 mL fluoride‐EDTA glucose (Sarstedt yellow top) Type and volume of sample: 1 mL CSF requested, minimum 400 µL required for analysis Specimen transport/special precautions: A CSF Xanthochromia sample collection kit should be used. -

Serum Total Protein, Albumin, Globulin Population of Various Ages and Sex in Karachi and Their Ratio in Apparently Healthy
SERUM TOTAL PROTEIN, ALBUMIN, GLOBULIN POPULATION OF VARIOUS AGES AND SEX IN KARACHI AND THEIR RATIO IN APPARENTLY HEALTHY Pages with reference to book, From 12 To 16 Khushnaseeb Ibrahim, Sarwar Jehan Zuberi ( PMRC Research Centre, Jinnah Postgraduate Medical Centre, Karachi. ) Syed Naznil Husnain ( Department of Biochemistry, University of Karachi, Karachi. ) Abstract Serum proteins, albumin and globulin in 456 (274 males and 182 females) subjects were within the normal range and showed unifornt distribution. Mean protein levels were low in infants under 0—1 year of age. No significant change was noted with age and sex in other groups. These values were considered to be normal for our population as all protein values fell within 2.5 and 97.5 percentile (95% of the total population) irrespective of age and sex except babies under 0—1 year. The average intake of protein/kg body weight was almost upto the recommended allowances(JPMA 39: 12, 1989). INTRODUCTION Serum protein levels in normals vary from 63—8 g% and albumin 4—6 g% 1-4 . This study repbrts levels of serum proteins, albumin, globulins and albumin/globulin ratios in healthy subjects and their relationship with age, sex and protein intake. SUBJECTS AND METHOD One thousand and thirty-six (526 males and 510 females) healthy subjects were selected from the hospital staff, well baby clinics, MCH Centres, primary and secondary schools, colleges and various organizations according to percentage distribution of age and sex in the Karachi population (Census, 1972). Information regarding age, sex, general physical health, dietary history, height and weight were recorded on a preceded proforma. -
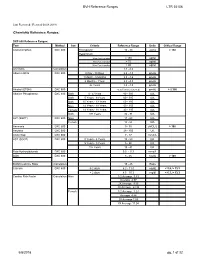
BVH Reference Ranges LTR 35106 Chemistry Reference Ranges
BVH Reference Ranges LTR 35106 Last Reviewed: (Revised 05.08.2018) Chemistry Reference Ranges: DXC 600 Reference Ranges: Test Method Sex Criteria Reference Range Units Critical Range Acetaminophen DXC 600 Therapeutic 10 - 30 ug/mL > 150 Hepatotoxic 4 hrs Post Ingestion > 150 ug/ml 8 hrs Post Ingestion > 75 ug/ml 12 hrs Post Ingestion > 40 ug/ml A/G Ratio Calculation 1.1 - 2.2 Albumin BCG DXC 600 0 Day - 30 Days 2.6 - 4.3 gm/dL 1 Month - 5 Months 2.8 - 4.6 gm/dL 6 Months - 1Year 2.8 - 4.8 gm/dL 2+ Years 3.2 - 4.9 gm/dL Alcohol (ETOH) DXC 600 <0.005 (none detected) gm/dL > 0.300 Alkaline Phosphatase DXC 600 Both 0 - 4 Years 80 - 350 IU/L Both 5 Years - 9 Years 60 - 385 IU/L Both 10 Years - 13 Years 60 - 485 IU/L Male 14 Years - 18 Years 50 - 350 IU/L Female 14 Years - 18 Years 40 - 195 IU/L Both 19+ Years 32 - 91 IU/L ALT (SGPT) DXC 600 Male 17 - 63 IU/L Female 14 - 54 IU/L Ammonia DXC 600 9 - 35 uMOL/L > 100 Amylase DXC 600 28 - 100 U/L Anion Gap DXC 600 7 - 17 mmol/L AST (SGOT) DXC 600 0 Years - 4 Years 10 - 60 IU/L 5 Years - 9 Years 5 - 50 IU/L 10+ Years 15 - 41 IU/L Beta Hydroxybutyrate DXC 600 0.0 - 0.3 mmol/L BUN DXC 600 8 - 26 mg/dL > 100 BUN/Creatinine Ratio Calculation 15 - 25 Ratio Calcium DXC 600 0-2 days 6.2 - 11.0 mg/dL < 5.8, > 13.3 > 2 days 8.5 - 10.3 mg/dl < 6.3, > 13.3 Cardiac Risk Factor Calculation Male 1/2 Average 3.43 Average 4.97 2X Average 9.55 3X Average 23.99 Female 1/2 Average 3.27 Average 4.44 2X Average 7.05 3X Average 11.04 5/8/2018 pg. -

Association Between Serum Liver Enzymes and Hypertension
Rahman et al. BMC Cardiovascular Disorders (2020) 20:128 https://doi.org/10.1186/s12872-020-01411-6 RESEARCH ARTICLE Open Access Association between serum liver enzymes and hypertension: a cross-sectional study in Bangladeshi adults Sadaqur Rahman, Shiful Islam, Tangigul Haque, Rahanuma Raihanu Kathak and Nurshad Ali* Abstract Background: Hypertension is a major contributing factor to cardiovascular disease and is a leading cause of death in the world. The association between hepatic enzymes and hypertension has been reported in limited studies and the findings are inconsistent; data from Bangladeshi adults are not available yet. This study was conducted to estimate the prevalence of elevated liver enzymes and evaluate the association of elevated liver enzymes with hypertension in Bangladeshi adults. Methods: In this cross-sectional study, 302 blood samples were collected from adult participants and analyzed the serum concentrations of alanine and aspartate aminotransferase (ALT, AST), alkaline phosphatase (ALP), and γ-glutamyltransferase (GGT) and other markers related to hypertension. Hypertension was defined as resting SBP ≥ 140 mmHg and/or DBP ≥ 90 mmHg. Associations between elevated liver enzymes and hypertension were evaluatedbymultinomiallogistic regression. Results: The mean concentrations of serum ALT, AST and GGT were significantly higher in the hypertensive group compared to the normotensive group (p < 0.01, p <0.01andp < 0.001, respectively). Overall, 49.2% of subjects in the hypertensive group and 38.1% of individuals in the normotensive group had at least one or more elevated liver enzymes. The prevalence of elevated ALT, AST, and GGT was significantly higher among participants in the hypertensive group compared to the normotensive group (p < 0.01, p <0.01andp < 0.001, respectively). -

Research Article Assessment of Diagnostic Accuracy and Optimal Cut Points of Blood Lead Levels on Serum Proteins Among Workers E
INTERNATIONAL JOURNAL OF MEDICAL BIOCHEMISTRY DOI: 10.14744/ijmb.2019.41736 Int J Med Biochem 2019;2(3):81-7 Research Article Assessment of diagnostic accuracy and optimal cut points of blood lead levels on serum proteins among workers exposed to Pb at a lead battery plant Ravibabu Kalahasthi, Tapu Barman, Ravi Prakash Jamalpur, Vinay Kumar Adepu Department of Biochemistry, Regional Occupational Health Centre (Southern), Bangalore, India Abstract Objectives: This study assessed the diagnostic accuracy and the optimal cut point of blood lead (Pb) level (BLL) in serum proteins (total protein, albumin, globulin, and albumin/globulin ratio) among workers with exposure to Pb at a Pb battery plant. Methods: An examination of the diagnostic accuracy and optimal cut point value of BLL in serum proteins among workers exposed to Pb was performed using analysis of the area under the curve (AUC) and coordinates of receiving operating characteristic (ROC) curve. The study group consisted of 176 males who worked in a Pb battery plant. A con- trol group consisted of 80 male office workers with no unusual occupational Pb exposure. The BLL of the patients was assessed using an atomic absorption spectrophotometer. The serum protein levels (total protein and albumin) were determined using diagnostic kits. The serum globulin was calculated from the total protein and albumin measures. The albumin:globulin ratio (A/G ratio) was calculated from the albumin and globulin levels. Results: The serum total protein and globulin concentration values were lower and the A/G ratio was significantly higher in the study group compared with the control group. A negative and significant association was found between the BLL and serum total protein and serum globulin values. -

Routine Cerebrospinal Fluid (CSF) Analysis
CHAPTER4 Routine cerebrospinal fluid (CSF) analysis a b a ) F. Deisenhammer, A. Bartos, R. Egg, Albumin CSF/serum ratio (Qalb should be pre- N. E. Gilhus,c G. Giovannoni,d S. Rauer,e ferred to total protein measurement and normal F. Sellebjergf upper limits should be related to patients’ age. Elevated Qalb is a non-specific finding but occurs mainly in bacterial, cryptococcal, and tubercu- Background A great variety of neurological diseases lous meningitis, leptomingeal metastases as well require investigation of the cerebrospinal fluid as acute and chronic demyelinating polyneu- (CSF) to prove the diagnosis or to rule out relevant ropathies. differential diagnoses. Pathological decrease of the CSF/serum glu- cose ratio or an increase in lactate concentration Objectives To evaluate the theoretical background indicates bacterial or fungal meningitis or lep- and provide guidelines for clinical use in rou- tomeningeal metastases. tine CSF analysis including total protein, albumin, Intrathecal immunoglobulin G synthesis is best immunoglobulins, glucose, lactate, cell count, demonstrated by isoelectric focusing followed by cytological staining, and investigation of infec- specific staining. tious CSF. Cellular morphology (cytological staining) should be evaluated whenever pleocytosis is found or Methods Systematic Medline search for the above leptomeningeal metastases or pathological bleed- mentioned variables. Review of appropriate publi- ing is suspected. Computed tomography-negative cations by one or more of the task force members. intrathecal bleeding should be investigated by Grading of evidence and recommendations was bilirubin detection. based on consensus by all task force members. Introduction CSF should be analysed immediately after collec- tion. If storage is needed 12 ml of CSF should The cerebrospinal fluid (CSF) is a dynamic, be partitioned into three to four sterile tubes. -
D40. Cerebrospinal Fluid.Pdf
CEREBROSPINAL FLUID D40 (1) Cerebrospinal Fluid (CSF) Last updated: June 3, 2019 PHYSIOLOGY ............................................................................................................................................ 1 CSF PRODUCTION .................................................................................................................................. 1 CSF REABSORPTION ............................................................................................................................... 1 PARAMETERS ........................................................................................................................................... 2 NORMAL ................................................................................................................................................ 2 OPENING PRESSURE ................................................................................................................................ 3 COLOR ................................................................................................................................................... 3 BLOODY CSF ......................................................................................................................................... 3 VISCOSITY & TURBIDITY ....................................................................................................................... 4 CELLS ................................................................................................................................................... -

Effects of Hyperglycemia and Rapid Lowering of Plasma Glucose in Normal Rabbits
MILESTONES IN NEPHROLOGY J Am Soc Nephrol 11: 1776–1788, 2000 Mark A. Knepper, Feature Editor Studies on Mechanisms of Cerebral Edema in Diabetic Comas EFFECTS OF HYPERGLYCEMIA AND RAPID LOWERING OF PLASMA GLUCOSE IN NORMAL RABBITS ALLEN I. ARIEFF AND CHARLES R. KLEEMAN WITH THE TECHNICAL ASSISTANCE OF ALICE KEUSHKERIAN AND HELEN BAGDOYAN From the Departments of Medicine, Wadsworth Veterans Administration Center and Cedars-Sinai Medical Center, and the Cedars-Sinai Medical Research Institute, and University of California Los Angeles Medical Center, Los Angeles, California 90048 with comments by ALLEN I. ARIEFF AND RICHARD STERNS Reprinted from J. Clin. Invest. 52:571–583, 1973 A BSTRACT To investigate the pathophysiology of cerebral edema occurring during treatment of diabetic coma, the effects of hyperglycemia and rapid lowering of plasma glucose were AUTHOR COMMENTARY evaluated in normal rabbits. During 2 h of hyperglycemia (plasma glucose = 61 mM), both brain (cerebral cortex) and Allen I. Arieff muscle initially lost about 10% of water content. After 4 h of hyperglycemia, skeletal muscle water content remained low University of California, but that of brain was normal. Brain osmolality (Osm) (343 San Francisco mosmol/kg H2O) was similar to that of cerebrospinal fluid Sausalito, California (CSF) (340 mosmol/kg), but increases in the concentration of Na+, K+, Cl–, glucose, sorbitol, lactate, urea, myoinositol, and amino acids accounted for only about half of this increase. n 1936, Dillon, Riggs, and Dyer described a syndrome The unidentified solute was designated “idiogenic osmoles”. Iwhereby individuals who were being treated for diabetic When plasma glucose was rapidly lowered to normal with coma and apparently recovering suddenly deteriorated, with insulin, there was gross brain edema, increases in brain content worsening of coma, respiratory insufficiency, hypotension, of water, Na+, K+, Cl– and idiogenic osmoles, and a signifi tachycardia, high fever, and death (1). -

Serial Estimation of Serum Albumin and Its Role in Traumatic Brain Injury Patients
ORIGINAL ARTICLE ASIAN JOURNAL OF MEDICAL SCIENCES Serial estimation of serum albumin and its role in traumatic brain injury patients Mithilesh Kumar Pandey1, Sunil Kumar Baranwal2, Deepak Singh Panwar3, Suniti Kumar Saha4, Kaushik Roy5, Subhasis Ghosh6, Parimal Tripathy7 1Assistant Professor, Department of Neurosurgery, Government Medical College Haldwani, Nainital, Uttarakhand, India, 2Post Doctoral Trainee Student, Department of Neurosurgery, Nil Ratan Sircar Medical College and Hospital, Kolkata, 3Consultant Neurosurgeon, Department of Neurosurgery, Government Medical College Haldwani, Nainital, Uttarakhand, India, 4Professor & HOD, Department of Neurosurgery, Nil Ratan Sircar Medical College and Hospital, Kolkata, 5Associate Professor, Department of Neurosurgery, Nil Ratan Sircar Medical College and Hospital, Kolkata, 6Professor, Department of Neurosurgery, Nil Ratan Sircar Medical College and Hospital, Kolkata, 7Former, HOD & Professor, Department of Neurosurgery, Nil Ratan Sircar Medical College and Hospital, Kolkata Submitted: 21-12-2015 Revised: 31-01-2016 Published: 01-07-2016 ABSTRACT Background: Serum albumin is routinely measured; cheap and easily available test even in Access this article online remote areas laboratories. Albumin is the major protein of human plasma and one of the Website: negative acute phase reactants reported to fall as a component of metabolic response to injury or infection, independent of the nutritional status. Even though this has also been http://nepjol.info/index.php/AJMS noted in patients with head injury and its overall signifi cance with respect to neurological DOI: 10.3126/ajms.v7i4.14142 outcome following head injury are yet to be established. Aims and Objectives: To assess E-ISSN: 2091-0576 P-ISSN: 2467-9100 the role of serum albumin in outcome of head injury patient.