Tests…But Maybe You Transports Don’T 2
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Thursday 23 June 2016 – Morning A2 GCE HUMAN BIOLOGY F225/01 Genetics, Control and Ageing *5884237032* Candidates Answer on the Question Paper
Oxford Cambridge and RSA Thursday 23 June 2016 – Morning A2 GCE HUMAN BIOLOGY F225/01 Genetics, Control and Ageing *5884237032* Candidates answer on the Question Paper. OCR supplied materials: Duration: 2 hours None Other materials required: • Electronic calculator • Ruler (cm/mm) *F22501* INSTRUCTIONS TO CANDIDATES • Write your name, centre number and candidate number in the boxes above. Please write clearly and in capital letters. • Use black ink. HB pencil may be used for graphs and diagrams only. • Answer all the questions. • Read each question carefully. Make sure you know what you have to do before starting your answer. • Write your answer to each question in the space provided. If additional space is required, you should use the lined page(s) at the end of this booklet. The question number(s) must be clearly shown. • Do not write in the bar codes. INFORMATION FOR CANDIDATES • The number of marks is given in brackets [ ] at the end of each question or part question. • The total number of marks for this paper is 100. • Where you see this icon you will be awarded marks for the quality of written communication in your answer. • You may use an electronic calculator. • You are advised to show all the steps in any calculations. • This document consists of 24 pages. Any blank pages are indicated. © OCR 2016 [K/500/8502] OCR is an exempt Charity DC (NH/SW) 119808/4 Turn over 2 Answer all the questions. 1 Excretion is the removal of metabolic waste products from the body. The kidney is one of the organs involved in excretion. -

Prenatal Growth Restriction, Retinal Dystrophy, Diabetes Insipidus and White Matter Disease: Expanding the Spectrum of PRPS1-Related Disorders
European Journal of Human Genetics (2015) 23, 310–316 & 2015 Macmillan Publishers Limited All rights reserved 1018-4813/15 www.nature.com/ejhg ARTICLE Prenatal growth restriction, retinal dystrophy, diabetes insipidus and white matter disease: expanding the spectrum of PRPS1-related disorders Almundher Al-Maawali1,2, Lucie Dupuis1, Susan Blaser3, Elise Heon4, Mark Tarnopolsky5, Fathiya Al-Murshedi2, Christian R Marshall6,7, Tara Paton6,7, Stephen W Scherer6,7 for the FORGE Canada Consortium9, Jeroen Roelofsen8, Andre´ BP van Kuilenburg8 and Roberto Mendoza-Londono*,1 PRPS1 codes for the enzyme phosphoribosyl pyrophosphate synthetase-1 (PRS-1). The spectrum of PRPS1-related disorders associated with reduced activity includes Arts syndrome, Charcot–Marie–Tooth disease-5 (CMTX5) and X-linked non-syndromic sensorineural deafness (DFN2). We describe a novel phenotype associated with decreased PRS-1 function in two affected male siblings. Using whole exome and Sanger sequencing techniques, we identified a novel missense mutation in PRPS1. The clinical phenotype in our patients is characterized by high prenatal maternal a-fetoprotein, intrauterine growth restriction, dysmorphic facial features, severe intellectual disability and spastic quadraparesis. Additional phenotypic features include macular coloboma-like lesions with retinal dystrophy, severe short stature and diabetes insipidus. Exome sequencing of the two affected male siblings identified a shared putative pathogenic mutation c.586C4T p.(Arg196Trp) in the PRPS1 gene that was maternally inherited. Follow-up testing showed normal levels of hypoxanthine in urine samples and uric acid levels in blood serum. The PRS activity was significantly reduced in erythrocytes of the two patients. Nucleotide analysis in erythrocytes revealed abnormally low guanosine triphosphate and guanosine diphosphate. -

Cerebrospinal Fluid in Critical Illness
Cerebrospinal Fluid in Critical Illness B. VENKATESH, P. SCOTT, M. ZIEGENFUSS Intensive Care Facility, Division of Anaesthesiology and Intensive Care, Royal Brisbane Hospital, Brisbane, QUEENSLAND ABSTRACT Objective: To detail the physiology, pathophysiology and recent advances in diagnostic analysis of cerebrospinal fluid (CSF) in critical illness, and briefly review the pharmacokinetics and pharmaco- dynamics of drugs in the CSF when administered by the intravenous and intrathecal route. Data Sources: A review of articles published in peer reviewed journals from 1966 to 1999 and identified through a MEDLINE search on the cerebrospinal fluid. Summary of review: The examination of the CSF has become an integral part of the assessment of the critically ill neurological or neurosurgical patient. Its greatest value lies in the evaluation of meningitis. Recent publications describe the availability of new laboratory tests on the CSF in addition to the conventional cell count, protein sugar and microbiology studies. Whilst these additional tests have improved our understanding of the pathophysiology of the critically ill neurological/neurosurgical patient, they have a limited role in providing diagnostic or prognostic information. The literature pertaining to the use of these tests is reviewed together with a description of the alterations in CSF in critical illness. The pharmacokinetics and pharmacodynamics of drugs in the CSF, when administered by the intravenous and the intrathecal route, are also reviewed. Conclusions: The diagnostic utility of CSF investigation in critical illness is currently limited to the diagnosis of an infectious process. Studies that have demonstrated some usefulness of CSF analysis in predicting outcome in critical illness have not been able to show their superiority to conventional clinical examination. -

Arterial Blood Gases: Acid-Base Balance
EDUCATIONAL COMMENTARY – ARTERIAL BLOOD GASES: ACID-BASE BALANCE Educational commentary is provided through our affiliation with the American Society for Clinical Pathology (ASCP). To obtain FREE CME/CMLE credits click on Earn CE Credits under Continuing Education on the left side of the screen. **Florida licensees, please note: This exercise will appear in CE Broker under the specialty of Blood Gas Analysis. LEARNING OUTCOMES On completion of this exercise, the participant should be able to • identify the important buffering systems in the human body. • explain the Henderson-Hasselbalch equation and its relationship to the bicarbonate/carbonic acid buffer system. • explain the different acid-base disorders, causes associated with them, and compensatory measures. • evaluate acid-base status using patient pH and pCO2 and bicarbonate levels. Introduction Arterial blood gas values are an important tool for assessing oxygenation and ventilation, evaluating acid- base status, and monitoring the effectiveness of therapy. The human body produces a daily net excess of acid through normal metabolic processes: cellular metabolism produces carbonic, sulfuric, and phosphoric acids. Under normal conditions, the body buffers accumulated hydrogen ions (H+) through a variety of buffering systems, the respiratory center, and kidneys to maintain a plasma pH of between 7.35 and 7.45. This tight maintenance of blood pH is essential: even slight changes in pH can alter the functioning of enzymes, the cellular uptake and use of metabolites, and the uptake and release of oxygen. Although diagnoses are made by physicians, laboratory professionals must be able to interpret arterial blood gas values to judge the validity of the laboratory results they report. -

CSF Xanthochromia
CSF Xanthochromia Pseudonyms – CSF bilirubin For investigation of suspected subarachnoid haemorrhage (SAH) in CT negative patients Xanthochromia is the yellow discoloration indicating the presence of bilirubin in CSF which appears as oxyhaemoglobin released from the breakdown of red blood cells following haemorrhage into the CSF is converted in vivo into bilirubin in a time‐dependent manner. A subarachnoid haemorrhage (SAH) is a spontaneous arterial bleeding into the subarachnoid space, usually from a cerebral aneurysm, and characterised by a severe sudden‐onset headache. The majority of positive cases are detected by computed tomography (CT) scanning but for those CT‐ negative patients presenting with a history suggestive of SAH the measurement of xanthochromia in CSF is advocated to detect those patients who actually have sustained a SAH and require treatment and to eliminate the possibility of SAH in the remainder without the need of confirmatory angiography. The CSF is collected by means of lumbar puncture (LP). For full interpretation of the result other CSF and blood tests must be collected at the same time – CSF protein and glucose; plasma glucose; and serum protein and bilirubin. General information CSF Xanthochromia sample collection kits are provided in the ACU (ORC) and AMU (Trafford) and also available from the Specimen Receptions at the laboratories at both sites. Concurrent samples should be requested for CSF protein and glucose, plasma glucose and serum LFTS (for protein and bilirubin) using the sample containers supplied. Collection container: CSF xanthochromia: White topped Universal container (In addition the following samples should be collected:‐ CSF glucose & protein: 1.2 mL fluoride‐EDTA glucose (Sarstedt yellow top) Serum protein & bilirubin: 4.9 mL SST (Sarstedt brown top) Plasma glucose: 2.7 mL fluoride‐EDTA glucose (Sarstedt yellow top) Type and volume of sample: 1 mL CSF requested, minimum 400 µL required for analysis Specimen transport/special precautions: A CSF Xanthochromia sample collection kit should be used. -

Endocrinology Test List Endocrinology Test List
For Endocrinologists Endocrinology Test List Endocrinology Test List Extensive Capabilities Managing patients with endocrine disorders is complex. Having access to the right test for the right patient is key. With a legacy of expertise in endocrine laboratory diagnostics, Quest Diagnostics offers an extensive menu of laboratory tests across the spectrum of endocrine disorders. This test list highlights the extensive menu of laboratory diagnostic tests we offer, including highly specialized tests and those performed using highly specific and sensitive mass spectrometry detection. It is conveniently organized by glandular function or common endocrine disorder, making it easy for you to identify the tests you need to care for the patients you treat. Comprehensive Care Quest Diagnostics Nichols Institute has been pioneering state-of-the-art endocrine testing for over four decades. Our commitment to innovative diagnostics and our dedication to quality and service means we deliver solutions that enable you to make informed clinical decisions for comprehensive patient management. We strive to remain at the forefront of innovation in endocrine testing so you can deliver the highest level of patient care. Abbreviations and Footnotes NDM, neonatal diabetes mellitus; MODY, maturity-onset diabetes of the young; CH, congenital hyperinsulinism; MSUD, maple syrup urine disease; IHH, idiopathic hypogonadotropic hypogonadism; BBS, Bardet-Biedl syndrome; OI, osteogenesis imperfecta; PKD, polycystic kidney disease; OPPG, osteoporosis-pseudoglioma syndrome; CPHD, combined pituitary hormone deficiency; GHD, growth hormone deficiency. The tests highlighted in green are performed using highly specific and sensitive mass spectrometry detection. Panels that include a test(s) performed using mass spectrometry are highlighted in yellow. For tests highlighted in blue, refer to the Athena Diagnostics website (athenadiagnostics.com/content/test-catalog) for test information. -

Diabetes Insipidus
Your feelings about Infertility conditions series: › Diabetes insipidus The Pituitary Foundation Information Booklets Working to support pituitary patients, their carers & families The Pituitary Foundation is a charity working About this booklet in the United Kingdom and Republic of The aim of this booklet is to provide information Ireland supporting patients with pituitary about diabetes insipidus. conditions, their carers, family and friends. You may find that not all of the information Our aims are to offer support through the applies to you in particular, but we hope it helps pituitary journey, provide information to the you to understand your condition better and community, and act as the patient voice to raise offers you a basis for discussion with your GP awareness and improve services. and endocrinologist. What is diabetes insipidus and why do we get it? 3 The two forms of diabetes insipidus 5 How is DI diagnosed and treated? 7 How is DI diagnosed? 7 What tests are carried out and how will they feel? 7 How is DI treated? 7 Aftercare 9 How will diabetes insipidus affect my life? 10 Prescriptions 10 Driving 10 Employment problems 10 Insurance & pensions 10 Personal medical identification 11 Toilet facilities card 11 National key scheme 11 Common questions 12-13 What DI means to me - a patient's story 14 Membership & donation information 15 2 Diabetes insipidus What is diabetes insipidus (DI) and why do we get it? Diabetes insipidus (DI) is caused by a problem with either the production, or action, of the hormone vasopressin (AVP). If you have DI your kidneys are unable to retain water. -

TITLE: Acid-Base Disorders PRESENTER: Brenda Suh-Lailam
TITLE: Acid-Base Disorders PRESENTER: Brenda Suh-Lailam Slide 1: Hello, my name is Brenda Suh-Lailam. I am an Assistant Director of Clinical Chemistry and Mass Spectrometry at Ann & Robert H. Lurie Children’s Hospital of Chicago, and an Assistant Professor of Pathology at Northwestern Feinberg School of Medicine. Welcome to this Pearl of Laboratory Medicine on “Acid-Base Disorders.” Slide 2: During metabolism, the body produces hydrogen ions which affect metabolic processes if concentration is not regulated. To maintain pH within physiologic limits, there are several buffer systems that help regulate hydrogen ion concentration. For example, bicarbonate, plasma proteins, and hemoglobin buffer systems. The bicarbonate buffer system is the major buffer system in the blood. Slide 3: In the bicarbonate buffer system, bicarbonate, which is the metabolic component, is controlled by the kidneys. Carbon dioxide is the respiratory component and is controlled by the lungs. Changes in the respiratory and metabolic components, as depicted here, can lead to a decrease in pH termed acidosis, or an increase in pH termed alkalosis. Slide 4: Because the bicarbonate buffer system is the major buffer system of blood, estimation of pH using the Henderson-Hasselbalch equation is usually performed, expressed as a ratio of bicarbonate and carbon dioxide. Where pKa is the pH at which the concentration of protonated and unprotonated species are equal, and 0.0307 is the solubility coefficient of carbon dioxide. Four variables are present in this equation; knowing three variables allows for calculation of the fourth. Since pKa is a constant, and pH and carbon dioxide are measured during blood gas analysis, bicarbonate can, therefore, be determined using this equation. -

Approach to Acute Kidney Injury: Differentiation of Prerenal, Postrenal and Intrinsic Renal Disease, Acute Tubular Necrosis and Renal Vascular Disease
Acute Kidney Injury, Renal Vascular Disease Renal Genital Urianry System 2019 APPROACH TO ACUTE KIDNEY INJURY: DIFFERENTIATION OF PRERENAL, POSTRENAL AND INTRINSIC RENAL DISEASE, ACUTE TUBULAR NECROSIS AND RENAL VASCULAR DISEASE Biff F. Palmer, MD, Office: H5.112; Phone 87848 Email: [email protected] LEARNING OBJECTIVES • Given laboratory tests results and radiographic imaging, be able to diagnose a patients with an increased serum creatinine concentration as having post-renal renal disease and list the common clinical etiologies of urinary obstruction • Given laboratory tests results and radiographic imaging, be able to diagnose a patients with an increased serum creatinine concentration as having pre-renal kidney injury • Given pertinent aspects of the history, physical examination, serum and urine electrolytes, and urinalysis in any patient, be able to distinguish pre-renal renal failure from intrinsic renal disease • Given urine and plasma concentrations of sodium and creatinine in any patient, be able to calculate the fractional excretion of sodium (FENa) and interpret the results • List the renal syndromes associated with use of NSAID’s • List the characteristics of thromboembolic disease of the kidney, multiple choesterol emboli syndrome, renal vein thrombosis, and renal artery stenosis After determining chronicity and assessing the level of renal function, one should attempt to classify the patient with renal disease into one of several syndromes based on the renal structures most affected: pre-renal, post-renal or intrinsic renal disease. This classification is based on the information obtained in the history, physical examination, laboratory tests and selected imaging studies. It is particularly important to identify prerenal and postrenal disorders because theses disorders are often readily reversible. -
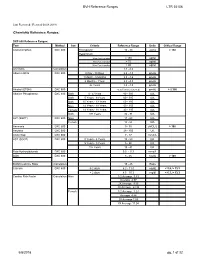
BVH Reference Ranges LTR 35106 Chemistry Reference Ranges
BVH Reference Ranges LTR 35106 Last Reviewed: (Revised 05.08.2018) Chemistry Reference Ranges: DXC 600 Reference Ranges: Test Method Sex Criteria Reference Range Units Critical Range Acetaminophen DXC 600 Therapeutic 10 - 30 ug/mL > 150 Hepatotoxic 4 hrs Post Ingestion > 150 ug/ml 8 hrs Post Ingestion > 75 ug/ml 12 hrs Post Ingestion > 40 ug/ml A/G Ratio Calculation 1.1 - 2.2 Albumin BCG DXC 600 0 Day - 30 Days 2.6 - 4.3 gm/dL 1 Month - 5 Months 2.8 - 4.6 gm/dL 6 Months - 1Year 2.8 - 4.8 gm/dL 2+ Years 3.2 - 4.9 gm/dL Alcohol (ETOH) DXC 600 <0.005 (none detected) gm/dL > 0.300 Alkaline Phosphatase DXC 600 Both 0 - 4 Years 80 - 350 IU/L Both 5 Years - 9 Years 60 - 385 IU/L Both 10 Years - 13 Years 60 - 485 IU/L Male 14 Years - 18 Years 50 - 350 IU/L Female 14 Years - 18 Years 40 - 195 IU/L Both 19+ Years 32 - 91 IU/L ALT (SGPT) DXC 600 Male 17 - 63 IU/L Female 14 - 54 IU/L Ammonia DXC 600 9 - 35 uMOL/L > 100 Amylase DXC 600 28 - 100 U/L Anion Gap DXC 600 7 - 17 mmol/L AST (SGOT) DXC 600 0 Years - 4 Years 10 - 60 IU/L 5 Years - 9 Years 5 - 50 IU/L 10+ Years 15 - 41 IU/L Beta Hydroxybutyrate DXC 600 0.0 - 0.3 mmol/L BUN DXC 600 8 - 26 mg/dL > 100 BUN/Creatinine Ratio Calculation 15 - 25 Ratio Calcium DXC 600 0-2 days 6.2 - 11.0 mg/dL < 5.8, > 13.3 > 2 days 8.5 - 10.3 mg/dl < 6.3, > 13.3 Cardiac Risk Factor Calculation Male 1/2 Average 3.43 Average 4.97 2X Average 9.55 3X Average 23.99 Female 1/2 Average 3.27 Average 4.44 2X Average 7.05 3X Average 11.04 5/8/2018 pg. -

Acid-Base Physiology & Anesthesia
ACID-BASE PHYSIOLOGY & ANESTHESIA Lyon Lee DVM PhD DACVA Introductions • Abnormal acid-base changes are a result of a disease process. They are not the disease. • Abnormal acid base disorder predicts the outcome of the case but often is not a direct cause of the mortality, but rather is an epiphenomenon. • Disorders of acid base balance result from disorders of primary regulating organs (lungs or kidneys etc), exogenous drugs or fluids that change the ability to maintain normal acid base balance. • An acid is a hydrogen ion or proton donor, and a substance which causes a rise in H+ concentration on being added to water. • A base is a hydrogen ion or proton acceptor, and a substance which causes a rise in OH- concentration when added to water. • Strength of acids or bases refers to their ability to donate and accept H+ ions respectively. • When hydrochloric acid is dissolved in water all or almost all of the H in the acid is released as H+. • When lactic acid is dissolved in water a considerable quantity remains as lactic acid molecules. • Lactic acid is, therefore, said to be a weaker acid than hydrochloric acid, but the lactate ion possess a stronger conjugate base than hydrochlorate. • The stronger the acid, the weaker its conjugate base, that is, the less ability of the base to accept H+, therefore termed, ‘strong acid’ • Carbonic acid ionizes less than lactic acid and so is weaker than lactic acid, therefore termed, ‘weak acid’. • Thus lactic acid might be referred to as weak when considered in relation to hydrochloric acid but strong when compared to carbonic acid. -

Hyperkalemia: This Time, Not the Usual Suspect
TUBULAR QUIZ Port J Nephrol Hypert 2018; 32(4): 395-397 • Advance Access publication 4 January 2019 Hyperkalemia: this time, not the usual suspect Anna Lima, Pedro Campos, Ana Gaspar, Afonso Santos, Patricia Carrilho Department of Nephrology, Hospital Prof Dr Fernando Fonseca, Lisbon, Portugal Received for publication: Dec 18, 2018 Accepted in revised form: Jan 4, 2019 A 49-year-old Caucasian male was admitted to the acidosis, one important step to search for the cause is emergency department with a three-day history of to calculate the anion gap.1 In this case the anion gap abdominal pain and nausea. These episodes were fre- was normal (AG= Na – (CL+HCO3) =12mmol/L). The next quent and, in the previous four months, he had noticed step was to calculate the urinary anion gap (UAG= (Na+ fatigue, more severe in the lower limbs, and symptoms + K+) – Cl-= 74mmol/L), which was highly positive. So, compatible with postural hypotension. He had no fever we were in presence of a metabolic acidosis with non- or any other symptoms. Lab tests requested by his increased anion gap, positive UAG, hyperkalemia and general practitioner six weeks earlier had revealed acidic urine (pH 5) in a patient with normal renal function hyperkalemia, without other relevant changes. He had matching a type IV (distal) renal tubular acidosis.2 followed a low-potassium diet since then. Past medical history included active smoking habits, intravenous There are many causes for hyperkalemic distal renal drug use (he suspended seventeen years ago), and tubular acidosis, the most common of which is diabetes changed bowel habits with alternating obstipation and mellitus (by definition a hyporeninemic hypoaldoster- diarrhea.