Hyperkalemia: This Time, Not the Usual Suspect
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Arterial Blood Gases: Acid-Base Balance
EDUCATIONAL COMMENTARY – ARTERIAL BLOOD GASES: ACID-BASE BALANCE Educational commentary is provided through our affiliation with the American Society for Clinical Pathology (ASCP). To obtain FREE CME/CMLE credits click on Earn CE Credits under Continuing Education on the left side of the screen. **Florida licensees, please note: This exercise will appear in CE Broker under the specialty of Blood Gas Analysis. LEARNING OUTCOMES On completion of this exercise, the participant should be able to • identify the important buffering systems in the human body. • explain the Henderson-Hasselbalch equation and its relationship to the bicarbonate/carbonic acid buffer system. • explain the different acid-base disorders, causes associated with them, and compensatory measures. • evaluate acid-base status using patient pH and pCO2 and bicarbonate levels. Introduction Arterial blood gas values are an important tool for assessing oxygenation and ventilation, evaluating acid- base status, and monitoring the effectiveness of therapy. The human body produces a daily net excess of acid through normal metabolic processes: cellular metabolism produces carbonic, sulfuric, and phosphoric acids. Under normal conditions, the body buffers accumulated hydrogen ions (H+) through a variety of buffering systems, the respiratory center, and kidneys to maintain a plasma pH of between 7.35 and 7.45. This tight maintenance of blood pH is essential: even slight changes in pH can alter the functioning of enzymes, the cellular uptake and use of metabolites, and the uptake and release of oxygen. Although diagnoses are made by physicians, laboratory professionals must be able to interpret arterial blood gas values to judge the validity of the laboratory results they report. -

TITLE: Acid-Base Disorders PRESENTER: Brenda Suh-Lailam
TITLE: Acid-Base Disorders PRESENTER: Brenda Suh-Lailam Slide 1: Hello, my name is Brenda Suh-Lailam. I am an Assistant Director of Clinical Chemistry and Mass Spectrometry at Ann & Robert H. Lurie Children’s Hospital of Chicago, and an Assistant Professor of Pathology at Northwestern Feinberg School of Medicine. Welcome to this Pearl of Laboratory Medicine on “Acid-Base Disorders.” Slide 2: During metabolism, the body produces hydrogen ions which affect metabolic processes if concentration is not regulated. To maintain pH within physiologic limits, there are several buffer systems that help regulate hydrogen ion concentration. For example, bicarbonate, plasma proteins, and hemoglobin buffer systems. The bicarbonate buffer system is the major buffer system in the blood. Slide 3: In the bicarbonate buffer system, bicarbonate, which is the metabolic component, is controlled by the kidneys. Carbon dioxide is the respiratory component and is controlled by the lungs. Changes in the respiratory and metabolic components, as depicted here, can lead to a decrease in pH termed acidosis, or an increase in pH termed alkalosis. Slide 4: Because the bicarbonate buffer system is the major buffer system of blood, estimation of pH using the Henderson-Hasselbalch equation is usually performed, expressed as a ratio of bicarbonate and carbon dioxide. Where pKa is the pH at which the concentration of protonated and unprotonated species are equal, and 0.0307 is the solubility coefficient of carbon dioxide. Four variables are present in this equation; knowing three variables allows for calculation of the fourth. Since pKa is a constant, and pH and carbon dioxide are measured during blood gas analysis, bicarbonate can, therefore, be determined using this equation. -

Approach to Acute Kidney Injury: Differentiation of Prerenal, Postrenal and Intrinsic Renal Disease, Acute Tubular Necrosis and Renal Vascular Disease
Acute Kidney Injury, Renal Vascular Disease Renal Genital Urianry System 2019 APPROACH TO ACUTE KIDNEY INJURY: DIFFERENTIATION OF PRERENAL, POSTRENAL AND INTRINSIC RENAL DISEASE, ACUTE TUBULAR NECROSIS AND RENAL VASCULAR DISEASE Biff F. Palmer, MD, Office: H5.112; Phone 87848 Email: [email protected] LEARNING OBJECTIVES • Given laboratory tests results and radiographic imaging, be able to diagnose a patients with an increased serum creatinine concentration as having post-renal renal disease and list the common clinical etiologies of urinary obstruction • Given laboratory tests results and radiographic imaging, be able to diagnose a patients with an increased serum creatinine concentration as having pre-renal kidney injury • Given pertinent aspects of the history, physical examination, serum and urine electrolytes, and urinalysis in any patient, be able to distinguish pre-renal renal failure from intrinsic renal disease • Given urine and plasma concentrations of sodium and creatinine in any patient, be able to calculate the fractional excretion of sodium (FENa) and interpret the results • List the renal syndromes associated with use of NSAID’s • List the characteristics of thromboembolic disease of the kidney, multiple choesterol emboli syndrome, renal vein thrombosis, and renal artery stenosis After determining chronicity and assessing the level of renal function, one should attempt to classify the patient with renal disease into one of several syndromes based on the renal structures most affected: pre-renal, post-renal or intrinsic renal disease. This classification is based on the information obtained in the history, physical examination, laboratory tests and selected imaging studies. It is particularly important to identify prerenal and postrenal disorders because theses disorders are often readily reversible. -

Acid-Base Physiology & Anesthesia
ACID-BASE PHYSIOLOGY & ANESTHESIA Lyon Lee DVM PhD DACVA Introductions • Abnormal acid-base changes are a result of a disease process. They are not the disease. • Abnormal acid base disorder predicts the outcome of the case but often is not a direct cause of the mortality, but rather is an epiphenomenon. • Disorders of acid base balance result from disorders of primary regulating organs (lungs or kidneys etc), exogenous drugs or fluids that change the ability to maintain normal acid base balance. • An acid is a hydrogen ion or proton donor, and a substance which causes a rise in H+ concentration on being added to water. • A base is a hydrogen ion or proton acceptor, and a substance which causes a rise in OH- concentration when added to water. • Strength of acids or bases refers to their ability to donate and accept H+ ions respectively. • When hydrochloric acid is dissolved in water all or almost all of the H in the acid is released as H+. • When lactic acid is dissolved in water a considerable quantity remains as lactic acid molecules. • Lactic acid is, therefore, said to be a weaker acid than hydrochloric acid, but the lactate ion possess a stronger conjugate base than hydrochlorate. • The stronger the acid, the weaker its conjugate base, that is, the less ability of the base to accept H+, therefore termed, ‘strong acid’ • Carbonic acid ionizes less than lactic acid and so is weaker than lactic acid, therefore termed, ‘weak acid’. • Thus lactic acid might be referred to as weak when considered in relation to hydrochloric acid but strong when compared to carbonic acid. -

A Case-Based Approach to Acid-Base Disorders
A Case-Based Approach to Acid-Base Disorders Justin Muir, PharmD Clinical Pharmacy Manager, Medical ICU NewYork-Presbyterian Hospital Columbia University Irving Medical Center [email protected] Disclosures None Objectives At the completion of this activity, pharmacists will be able to: 1. Describe acid-base physiology and disease states that lead to acid-base disorders. 2. Demonstrate a step-wise approach to interpretation of acid-base disorders and compensatory states. 3. Analyze contemporary literature regarding the use of sodium bicarbonate in metabolic acidosis. At the completion of this activity, pharmacy technicians will be able to: 1. Explain the importance of acid-base balance. 2. List the acid-base disorders seen in clinical practice. 3. Identify potential therapies used to treat acid-base disorders. Case A 51 year old man with history of erosive esophagitis, diabetes mellitus, chronic pancreatitis, and bipolar disorder is admitted with several days of severe nausea, vomiting, and abdominal pain. 135 87 31 pH 7.46 / pCO 29 / pO 81 861 2 2 BE -3.8 / HCO - 18 / SaO 96 5.6 20 0.9 3 2 • What additional data should be obtained? • What acid base disturbance(s) is/are present? Introduction • Acid base status is tightly regulated to maintain normal biochemical reactions and organ function • Body uses multiple mechanisms to maintain homeostasis • Abnormalities are extremely common in hospitalized patients with a higher incidence in critically ill with more complex pictures • A standard approach to analysis can help guide diagnosis and treatment Important acid-base determinants Blood gas generally includes at least: Normal range Measurement Description (arterial blood) pH -log [H+] 7.35-7.45 pCO2 partial pressure of dissolved CO2 35-45 mmHg pO2 partial pressure of dissolved O2 80-100 mmHg Base excess calculated measure of metabolic acid/base deviation from normal -3 to +3 SO2 calculated measure of Hgb O2 saturation based on pO2 95-100% - HCO3 calculated measure based on relationship of pH and pCO2 22-26 mEq/L Haber RJ. -

Acid-Base Balance
Intensive Care Nursery House Staff Manual Acid-Base Balance INTRODUCTION: The newborn infant is subject to numerous conditions that may disturb acid-base homeostasis. Management of ventilation, which controls the respiratory component of acid-base balance, is discussed in the section on Respiratory Support (P. 10). This section is a brief discussion of the metabolic aspects of acid-base balance. METABOLIC ACIDOSIS, defined as a base deficit >5 mEq/L on the first day and >4 mEq/L thereafter, occurs from: •Loss of buffer (mainly bicarbonate) or •Excess production of acid or decreased excretion of acid The anion gap is a useful calculation in assessing metabolic acidosis. + - - Anion gap = [Na ] – ([Cl ] + [HCO3 ]) Loss of buffer has no effect on anion gap. Accumulation of organic acid (e.g., lactic acid) causes an increase in anion gap. Normal anion gap: <15 mEq/L Increased anion gap: >15 mEq/L in LBW infants (<2,500 g) >18 mEq/L in ELBW infants (<1,000 g) Newborn infants normally have a base deficit of 1 to 3 mEq/L. Common causes of metabolic acidosis: •Bicarbonate loss, especially via immature kidney or from GI tract •Lactic acidosis from inadequate tissue perfusion and oxygenation (e.g., from asphyxia, shock, severe anemia, hypoxemia, PDA, NEC, excessive ventilator pressures with ↓ cardiac output) •Hypothermia •Organic acidemia due to an inborn error of metabolism (see P. 155) •Excessive Cl in IV fluids •Renal failure •Excessive acid load from high protein formula in preterm (late metabolic acidosis of prematurity ) - •Excretion of HCO3 as metabolic compensation for respiratory alkalosis Dilution acidosis is caused by excessive volume expansion (with saline, Ringer’s lactate or dextrose solutions). -

Tests…But Maybe You Transports Don’T 2
11/12/15 Some things you should When lab tests are useful know about laboratory 1. Managing patients during critical care tests…But maybe you transports don’t 2. While transporting patient to medical facilities for evaluation of laboratory Steve Faynor, CCEMT-P abnormalities HCA Chippenham Medical Center Richmond Ambulance Authority Objectives 1. Review some basic laboratory tests. Treat the patient, not the 2. Appreciate how patterns of laboratory test results can offer insight into etiology. laboratory values. 3. Learn how laboratory test calculations can add additional clinical information. 4. Review some limitations of laboratory tests. ELECTROLYTES & A case of “bad labs” RENAL FUNCTION TESTS 1 11/12/15 Hypernatremia & Renal Failure Hypernatremia • 89 year old white female • Hyperaldosteronism • Coming from nursing home due to • Cushing’s disease or syndrome abnormal labs • Diabetes insipidus (deficiency of ADH) • Sodium 172 mmol/L • Dehydration • Potassium 4.2 mmol/L • Chloride 137 mmol/L • Carbon dioxide 21 mmol/L • What are some causes of hypernatremia? • BP 122/66, SBP 99 later • BUN 212 mg N/dL • HR 64/min • Creatinine 6.10 mg/dL • RR 21/min • What do these values indicate? • SpCO2 98% on 4 L oxygen per min • Does this change your therapy? • Tongue dry, skin turgor poor • What is the cause of the hypernatremia in this patient? Treatment? Acute Renal Failure Use of the BUN/creatinine ratio • Intrinsic renal disease • In intrinsic causes of acute renal failure, the – Acute tubular necrosis: ischemia, toxins BUN/creatinine ratio is typically 10-15. – Acute glomerulonephritis • In pre-renal causes of acute renal failure, – CKD with missed dialysis the BUN/creatinine ratio is typically >20. -

Neurologic Complications of Electrolyte Disturbances and Acid–Base Balance
Handbook of Clinical Neurology, Vol. 119 (3rd series) Neurologic Aspects of Systemic Disease Part I Jose Biller and Jose M. Ferro, Editors © 2014 Elsevier B.V. All rights reserved Chapter 23 Neurologic complications of electrolyte disturbances and acid–base balance ALBERTO J. ESPAY* James J. and Joan A. Gardner Center for Parkinson’s Disease and Movement Disorders, Department of Neurology, UC Neuroscience Institute, University of Cincinnati, Cincinnati, OH, USA INTRODUCTION hyperglycemia or mannitol intake, when plasma osmolal- ity is high (hypertonic) due to the presence of either of The complex interplay between respiratory and renal these osmotically active substances (Weisberg, 1989; function is at the center of the electrolytic and acid-based Lippi and Aloe, 2010). True or hypotonic hyponatremia environment in which the central and peripheral nervous is always due to a relative excess of water compared to systems function. Neurological manifestations are sodium, and can occur in the setting of hypovolemia, accompaniments of all electrolytic and acid–base distur- euvolemia, and hypervolemia (Table 23.2), invariably bances once certain thresholds are reached (Riggs, reflecting an abnormal relationship between water and 2002). This chapter reviews the major changes resulting sodium, whereby the former is retained at a rate faster alterations in the plasma concentration of sodium, from than the latter (Milionis et al., 2002). Homeostatic mech- potassium, calcium, magnesium, and phosphorus as well anisms protecting against changes in volume and sodium as from acidemia and alkalemia (Table 23.1). concentration include sympathetic activity, the renin– angiotensin–aldosterone system, which cause resorption HYPONATREMIA of sodium by the kidneys, and the hypothalamic arginine vasopressin, also known as antidiuretic hormone (ADH), History and terminology which prompts resorption of water (Eiskjaer et al., 1991). -

ACS/ASE Medical Student Core Curriculum Acid-Base Balance
ACS/ASE Medical Student Core Curriculum Acid-Base Balance ACID-BASE BALANCE Epidemiology/Pathophysiology Understanding the physiology of acid-base homeostasis is important to the surgeon. The two acid-base buffer systems in the human body are the metabolic system (kidneys) and the respiratory system (lungs). The simultaneous equilibrium reactions that take place to maintain normal acid-base balance are: H" HCO* ↔ H CO ↔ H O l CO g To classify the type of disturbance, a blood gas (preferably arterial) and basic metabolic panel must be obtained. A basic understanding of normal acid-base buffer physiology is required to understand alterations in these labs. The normal pH of human blood is 7.40 (7.35-7.45). This number is tightly regulated by the two buffer systems mentioned above. The lungs contain carbonic anhydrase which is capable of converting carbonic acid to water and CO2. The respiratory response results in an alteration to ventilation which allows acid to be retained or expelled as CO2. Therefore, bradypnea will result in respiratory acidosis while tachypnea will result in respiratory alkalosis. The respiratory buffer system is fast acting, resulting in respiratory compensation within 30 minutes and taking approximately 12 to 24 hours to reach equilibrium. The renal metabolic response results in alterations in bicarbonate excretion. This system is more time consuming and can typically takes at least three to five days to reach equilibrium. Five primary classifications of acid-base imbalance: • Metabolic acidosis • Metabolic alkalosis • Respiratory acidosis • Respiratory alkalosis • Mixed acid-base disturbance It is important to remember that more than one of the above processes can be present in a patient at any given time. -

Renal-Tubular-Acidosis-NAGMA
Non-Anion Gap Metabolic Acidosis App.GoSoapbox.com 665-971-584 then “Join Now” Joel M. Topf, M.D. http://PBFluids.com @kidney_boy App.GoSoapbox.com 665-971-584 32 y.o. female with fatigue, weakness and muscle aches App.GoSoapbox.com 665-971-584 Answer polls: • Uhura: What is the primary acid base… • Uhura: What is the appropriate pCO2… 139 115 16 7.34 / 33 / 87 / 18 3.1 17 1.0 32 y.o. female with fatigue, weakness and muscle aches 139 115 16 7.34 / 33 / 87 / 18 3.1 17 1.0 Determine the primary disorder 7.34 / 87 / 33 / 16 pH / pO2 / pCO2 / HCO3 1. Acidosis or alkalosis – If thethe pHpH is is less less than than 7.4 7.4 it it is is acidosis acidosis – If the pH is greater than 7.4 it is alkalosis 2. DetermineMetabolic if it is respiratory or metabolic Acidosis – If thethe pH,pH, bicarbonate bicarbonate and and pCO pCO2 all2 allmove move in thein thesame same direction direction (up or(up down)or down) it is it metabolic is metabolic – If the pH, bicarbonate and pCO2 move in discordant directions (up and down)and down) it is respiratoryit is respiratory Predicting pCO2 in metabolic acidosis: Winter’s Formula • In metabolic acidosis the expected pCO2 can be estimated from the HCO3 Expected pCO2 = (1.5 x HCO3) + 8 ± 2 • If the pCO2 is higher than predicted then there is an addition respiratory acidosis • If the pCO2 is lower than predicted there is an additional respiratory alkalosis Is the compensation appropriate? 7.33 / 87 / 33 / 17 pH / pO2 / pCO2 / HCO3 ± Appropriately• Expected pCO2 = (1.5 x HCOcompensated3) + 8 2 • Expected pCO2 = 31-35 metabolic acidosis • Actual pCO2 is 33, which is within the predicted range, indicating appropriate respiratory compensation What anion is associated with the additional acid? • Metabolic acidosis is further evaluated by determining the anion associated with the increased H+ cation • In medicine we categorize the anions into: chloride not chloride Non-Anion Gap Anion Gap Metabolic Acidosis Metabolic Acidosis …and differentiate the two based on the anion gap. -

Acid Base Interpretation
Clinical Guidance Paediatric Critical Care: Acid Base Interpretation Summary This guideline is for the use by clinical staff who are interpreting acid base balance in critical care. Rather than focusing on bicarbonate which is a derived value, interpretation is aided by accounting for the contribution made to acid-base by: chloride, albumin and unmeasured acids. Document Detail Document type Clinical Guideline Document name Paediatric Critical Care: Acid Base Interpretation Document location GTi Clinical Guidance Database & Evelina London website Version 2 Effective from April 2018 Review date April 2021 Owner PICU Head of Service Author(s) Jon Lillie, PICU Consultant (original by Andrew Durward) Approved by, date Evelina London Clinical Guidelines Group, April 2018 Superseded documents Related documents Keywords Evelina, child, Paediatric, intensive care, STRS, Retrieval, Paediatric critical care, Acid Base Interpretation, PICU Relevant external law, regulation, standards This clinical guideline has been produced by the South Thames Retrieval Service (STRS) at Evelina London for nurses, doctors and ambulance staff to refer to in the emergency care of critically ill children. This guideline represents the views of STRS and was produced after careful consideration of available evidence in conjunction with clinical expertise and experience. The guidance does not override the individual responsibility of healthcare professionals to make decisions appropriate to the circumstances of the individual patient. Change History Date Change details, since approval Approved by Paediatric Critical Care Acid base interpretation Traditional teaching relies on Hendersen-Hasselbalch formula that states HCO3 (metabolic component) and pCO2 (respiratory component) can vary independently according to the formula pH = 6.1 + (HCO3/pCO2 mmHg x 0.003) This assumes bicarbonate is the major and only significant buffer for acidosis. -
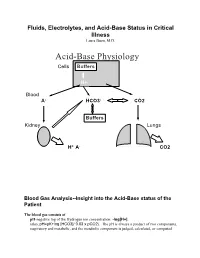
Acid-Base Physiology Cells Buffers
Fluids, Electrolytes, and Acid-Base Status in Critical Illness Laura Ibsen, M.D. Acid-Base Physiology Cells Buffers H+ Blood A- HCO3- CO2 Buffers Kidney Lungs H+ A- CO2 Blood Gas Analysis--Insight into the Acid-Base status of the Patient The blood gas consists of pH-negative log of the Hydrogen ion concentration: -log[H+]. (also, pH=pK+log [HCO3]/ 0.03 x pCO2). The pH is always a product of two components, respiratory and metabolic, and the metabolic component is judged, calculated, or computed by allowing for the effect of the pCO2, ie, any change in the pH unexplained by the pCO2 indicates a metabolic abnormality. - + CO2+H20º H2CO3ºHCO3 + H CO2 and water form carbonic acid or H2CO3, which is in equilibrium with bicarbonate (HCO3-)and hydrogen ions (H+). A change in the concentration of the reactants on either side of the equation affects the subsequent direction of the reaction. For example, an increase in CO2 will result in increased carbonic acid formation (H2CO3) which leads to an increase in both HCO3- and H+ (\pH). Normally, at pH 7.4, a ratio of one part carbonic acid to twenty parts bicarbonate is present in the extracellular fluid [HCO3-/H2CO3]=20. A change in the ratio will affect the pH of the fluid. If both components change (ie, with chronic compensation), the pH may be normal, but the other components will not. pCO2-partial pressure of carbon dioxide. Hypoventilation or hyperventilation (ie, minute ventilation--tidal volume x respitatory rate--imperfectly matched to physiologic demands) will lead to elevation or depression, respectively, in the pCO2.