Routine Cerebrospinal Fluid (CSF) Analysis
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Cerebrospinal Fluid in Critical Illness
Cerebrospinal Fluid in Critical Illness B. VENKATESH, P. SCOTT, M. ZIEGENFUSS Intensive Care Facility, Division of Anaesthesiology and Intensive Care, Royal Brisbane Hospital, Brisbane, QUEENSLAND ABSTRACT Objective: To detail the physiology, pathophysiology and recent advances in diagnostic analysis of cerebrospinal fluid (CSF) in critical illness, and briefly review the pharmacokinetics and pharmaco- dynamics of drugs in the CSF when administered by the intravenous and intrathecal route. Data Sources: A review of articles published in peer reviewed journals from 1966 to 1999 and identified through a MEDLINE search on the cerebrospinal fluid. Summary of review: The examination of the CSF has become an integral part of the assessment of the critically ill neurological or neurosurgical patient. Its greatest value lies in the evaluation of meningitis. Recent publications describe the availability of new laboratory tests on the CSF in addition to the conventional cell count, protein sugar and microbiology studies. Whilst these additional tests have improved our understanding of the pathophysiology of the critically ill neurological/neurosurgical patient, they have a limited role in providing diagnostic or prognostic information. The literature pertaining to the use of these tests is reviewed together with a description of the alterations in CSF in critical illness. The pharmacokinetics and pharmacodynamics of drugs in the CSF, when administered by the intravenous and the intrathecal route, are also reviewed. Conclusions: The diagnostic utility of CSF investigation in critical illness is currently limited to the diagnosis of an infectious process. Studies that have demonstrated some usefulness of CSF analysis in predicting outcome in critical illness have not been able to show their superiority to conventional clinical examination. -

CSF Xanthochromia
CSF Xanthochromia Pseudonyms – CSF bilirubin For investigation of suspected subarachnoid haemorrhage (SAH) in CT negative patients Xanthochromia is the yellow discoloration indicating the presence of bilirubin in CSF which appears as oxyhaemoglobin released from the breakdown of red blood cells following haemorrhage into the CSF is converted in vivo into bilirubin in a time‐dependent manner. A subarachnoid haemorrhage (SAH) is a spontaneous arterial bleeding into the subarachnoid space, usually from a cerebral aneurysm, and characterised by a severe sudden‐onset headache. The majority of positive cases are detected by computed tomography (CT) scanning but for those CT‐ negative patients presenting with a history suggestive of SAH the measurement of xanthochromia in CSF is advocated to detect those patients who actually have sustained a SAH and require treatment and to eliminate the possibility of SAH in the remainder without the need of confirmatory angiography. The CSF is collected by means of lumbar puncture (LP). For full interpretation of the result other CSF and blood tests must be collected at the same time – CSF protein and glucose; plasma glucose; and serum protein and bilirubin. General information CSF Xanthochromia sample collection kits are provided in the ACU (ORC) and AMU (Trafford) and also available from the Specimen Receptions at the laboratories at both sites. Concurrent samples should be requested for CSF protein and glucose, plasma glucose and serum LFTS (for protein and bilirubin) using the sample containers supplied. Collection container: CSF xanthochromia: White topped Universal container (In addition the following samples should be collected:‐ CSF glucose & protein: 1.2 mL fluoride‐EDTA glucose (Sarstedt yellow top) Serum protein & bilirubin: 4.9 mL SST (Sarstedt brown top) Plasma glucose: 2.7 mL fluoride‐EDTA glucose (Sarstedt yellow top) Type and volume of sample: 1 mL CSF requested, minimum 400 µL required for analysis Specimen transport/special precautions: A CSF Xanthochromia sample collection kit should be used. -
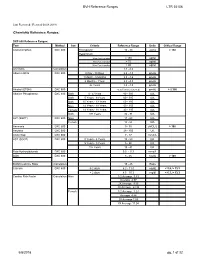
BVH Reference Ranges LTR 35106 Chemistry Reference Ranges
BVH Reference Ranges LTR 35106 Last Reviewed: (Revised 05.08.2018) Chemistry Reference Ranges: DXC 600 Reference Ranges: Test Method Sex Criteria Reference Range Units Critical Range Acetaminophen DXC 600 Therapeutic 10 - 30 ug/mL > 150 Hepatotoxic 4 hrs Post Ingestion > 150 ug/ml 8 hrs Post Ingestion > 75 ug/ml 12 hrs Post Ingestion > 40 ug/ml A/G Ratio Calculation 1.1 - 2.2 Albumin BCG DXC 600 0 Day - 30 Days 2.6 - 4.3 gm/dL 1 Month - 5 Months 2.8 - 4.6 gm/dL 6 Months - 1Year 2.8 - 4.8 gm/dL 2+ Years 3.2 - 4.9 gm/dL Alcohol (ETOH) DXC 600 <0.005 (none detected) gm/dL > 0.300 Alkaline Phosphatase DXC 600 Both 0 - 4 Years 80 - 350 IU/L Both 5 Years - 9 Years 60 - 385 IU/L Both 10 Years - 13 Years 60 - 485 IU/L Male 14 Years - 18 Years 50 - 350 IU/L Female 14 Years - 18 Years 40 - 195 IU/L Both 19+ Years 32 - 91 IU/L ALT (SGPT) DXC 600 Male 17 - 63 IU/L Female 14 - 54 IU/L Ammonia DXC 600 9 - 35 uMOL/L > 100 Amylase DXC 600 28 - 100 U/L Anion Gap DXC 600 7 - 17 mmol/L AST (SGOT) DXC 600 0 Years - 4 Years 10 - 60 IU/L 5 Years - 9 Years 5 - 50 IU/L 10+ Years 15 - 41 IU/L Beta Hydroxybutyrate DXC 600 0.0 - 0.3 mmol/L BUN DXC 600 8 - 26 mg/dL > 100 BUN/Creatinine Ratio Calculation 15 - 25 Ratio Calcium DXC 600 0-2 days 6.2 - 11.0 mg/dL < 5.8, > 13.3 > 2 days 8.5 - 10.3 mg/dl < 6.3, > 13.3 Cardiac Risk Factor Calculation Male 1/2 Average 3.43 Average 4.97 2X Average 9.55 3X Average 23.99 Female 1/2 Average 3.27 Average 4.44 2X Average 7.05 3X Average 11.04 5/8/2018 pg. -

Basic Skills in Interpreting Laboratory Data, 5Th Edition
CHAPTER 1 DEFINITIONS AND CONCEPTS KAREN J. TIETZE This chapter is based, in part, on the second edition chapter titled “Definitions and Concepts,” which was written by Scott L. Traub. Objectives aboratory testing is used to detect disease, guide treatment, monitor response Lto treatment, and monitor disease progression. However, it is an imperfect sci ence. Laboratory testing may fail to identify abnormalities that are present (false After completing this chapter, negatives [FNs]) or identify abnormalities that are not present (false positives, the reader should be able to [FPs]). This chapter defines terms used to describe and differentiate laboratory • Differentiate between accuracy tests and describes factors that must be considered when assessing and applying and precision laboratory test results. • Distinguish between quantitative, qualitative, and semiqualitative DEFINITIONS laboratory tests Many terms are used to describe and differentiate laboratory test characteristics and • Define reference range and identify results. The clinician should recognize and understand these terms before assessing factors that affect a reference range and applying test results to individual patients. • Differentiate between sensitivity and Accuracy and Precision specificity, and calculate and assess Accuracy and precision are important laboratory quality control measures. Labora these parameters tories are expected to test analytes with accuracy and precision and to document the • Identify potential sources of quality control procedures. Accuracy of a quantitative assay is usually measured in laboratory errors and state the terms of an analytical performance, which includes accuracy and precision. Accuracy impact of these errors in the is defined as the extent to which the mean measurement is close to the true value. -

Ascites and Related Disorders
AscitesAscites andand RelatedRelated DisordersDisorders LuisLuis S.S. Marsano,Marsano, MDMD ProfessorProfessor ofof MedicineMedicine DirectorDirector ofof HepatologyHepatology UniversityUniversity ofof LouisvilleLouisville CausesCauses ofof AscitesAscites Malignant Neoplasia 10% Cardiac Insufficiency 3% Tuberculous Peritonitis Chronic hepatic 2% disease Nephrogenic 81% a scite s (dia lysis) 1% Pancreatic ascites 1% Biliary ascites 1% Others 1% PathophysiologyPathophysiology ofof CirrhoticCirrhotic AscitesAscites Hepatic sinusoidal pressure Activation of hepatic baroreceptors Compensated Peripheral arterial vasodilation with hypervolemia, (normal renin, aldosterone, vasopressin, or norepinephrine) Peripheral arterial vasodilation (“underfilling”) Decompensated Neurally mediated Na+ retention, (with elevated renin, aldosterone, vasopressin, or norepinephrine) ClassificationClassification ofof AscitesAscites SerumSerum--ascitesascites albuminalbumin gradientgradient (SAAG)(SAAG) SAAGSAAG (g/dl)(g/dl) == albuminalbumins –– albuminalbumina GradientGradient >>1.11.1 g/dlg/dl == portalportal hypertensionhypertension Serum globulin > 5 g/dl:: – SAAG correction = (SAAG mean)(0.21+0.208 serum globulin g/dl) AscitesAscites withwith HighHigh SAAGSAAG >>1.11.1 g/dlg/dl == portalportal hypertensionhypertension Cirrhosis Alcoholic Hepatitis Cardiac ascites Massive hepatic metastasis Fulminant hepatic failure Budd-Chiari syndrome Portal vein thrombosis Veno-occlusive disease Acute fatty liver of pregnancy Myxedema Mixed ascites LowLow SAAGSAAG <1.1<1.1 -

Hyperchloremia – Why and How
Document downloaded from http://www.elsevier.es, day 23/05/2017. This copy is for personal use. Any transmission of this document by any media or format is strictly prohibited. n e f r o l o g i a 2 0 1 6;3 6(4):347–353 Revista de la Sociedad Española de Nefrología www.revistanefrologia.com Brief review Hyperchloremia – Why and how Glenn T. Nagami Nephrology Section, Department of Medicine, VA Greater Los Angeles Healthcare System and David Geffen School of Medicine at UCLA, United States a r t i c l e i n f o a b s t r a c t Article history: Hyperchloremia is a common electrolyte disorder that is associated with a diverse group of Received 5 April 2016 clinical conditions. The kidney plays an important role in the regulation of chloride concen- Accepted 11 April 2016 tration through a variety of transporters that are present along the nephron. Nevertheless, Available online 3 June 2016 hyperchloremia can occur when water losses exceed sodium and chloride losses, when the capacity to handle excessive chloride is overwhelmed, or when the serum bicarbonate is low Keywords: with a concomitant rise in chloride as occurs with a normal anion gap metabolic acidosis Hyperchloremia or respiratory alkalosis. The varied nature of the underlying causes of the hyperchloremia Electrolyte disorder will, to a large extent, determine how to treat this electrolyte disturbance. Serum bicarbonate Published by Elsevier Espana,˜ S.L.U. on behalf of Sociedad Espanola˜ de Nefrologıa.´ This is an open access article under the CC BY-NC-ND license (http://creativecommons.org/ licenses/by-nc-nd/4.0/). -

1 Molecular Physiology and Pathophysiology of Bilirubin Handling by the Blood, Liver
1 1 MOLECULAR PHYSIOLOGY AND PATHOPHYSIOLOGY OF BILIRUBIN HANDLING BY THE BLOOD, LIVER, 2 INTESTINE, AND BRAIN IN THE NEWBORN 3 THOR W.R. HANSEN1, RONALD J. WONG2, DAVID K. STEVENSON2 4 1Division of Paediatric and Adolescent Medicine, Institute of Clinical Medicine, Faculty of Medicine, 5 University of Oslo, Norway 6 2Department of Pediatrics, Stanford University School of Medicine, Stanford CA, USA 7 __________________________________________________________________________________ 8 2 9 I. Introduction 10 II. Bilirubin in the Body 11 A. Bilirubin Chemistry 12 1. Bilirubin structure 13 2. Bilirubin solubility 14 3. Bilirubin isomers 15 4. Heme degradation 16 5. Biliverdin and biliverdin reductase (BVR) 17 B. Bilirubin as an Antioxidant 18 C. Bilirubin as a Toxin 19 1. Bilirubin effects on enzyme activity 20 2. Toxicity of bilirubin conjugates and isomers 21 D. Other Functions/Roles 22 1. Drug displacement by bilirubin 23 2. Bilirubin interactions with the immune system and 24 inflammatory/infectious mechanisms 25 III. The Production of Bilirubin in the Body 26 A. Heme Catabolism and Its Regulation 27 1. Genetic variants in bilirubin production 28 B. The Effect of Hemolysis 29 1. Disorders associated with increased bilirubin production 30 IV. Bilirubin Binding and Transport in Blood 31 V. Bilirubin in the Liver 32 A. Hepatocellular Uptake and Intracellular Processing 33 B. Bilirubin Conjugation 34 1. Genetic variants in bilirubin conjugation 3 35 a. Crigler-Najjar syndrome type I 36 b. Crigler-Najjar syndrome type II 37 c. Gilbert syndrome 38 2. Genetic variants in transporter proteins 39 C. Bilirubin Excretion 40 VI. Bilirubin in the Intestines 41 A. -
D40. Cerebrospinal Fluid.Pdf
CEREBROSPINAL FLUID D40 (1) Cerebrospinal Fluid (CSF) Last updated: June 3, 2019 PHYSIOLOGY ............................................................................................................................................ 1 CSF PRODUCTION .................................................................................................................................. 1 CSF REABSORPTION ............................................................................................................................... 1 PARAMETERS ........................................................................................................................................... 2 NORMAL ................................................................................................................................................ 2 OPENING PRESSURE ................................................................................................................................ 3 COLOR ................................................................................................................................................... 3 BLOODY CSF ......................................................................................................................................... 3 VISCOSITY & TURBIDITY ....................................................................................................................... 4 CELLS ................................................................................................................................................... -

2017 LAB GUIDE TEST MENU March 2017Edition
2017 LAB GUIDE TEST MENU March 2017Edition Replaces: Sept 2015 Edition STAT TESTING MENU (2 Pages) 30 Minute In-Lab Turnaround Time (TAT) Unless Further Noted Below (In-Lab Time) MICROBIOLOGY 1. Spinal Fluid Culture set up and Gram Stain of sediment, India Ink prep 2. Gram Stain, other sites 3. Planting of cultures 4. Wet prep 5. Rapid Strep A Antigen Test (15 min. TAT) 6. Rapid Influenza A & B Antigen test 7. Rapid RSV Antigen test 8. Malaria Prep (60 min. TAT), Evening and Night shifts will only report “Parasites present or absent” 9. Screen, Gastric Urease, for Helicobacter Pylori (60 min. TAT) 10. Clostridium difficile DNA amplification (60 min. TAT) BLOOD BANK 1. Compatibility testing (leuko-reduced packed cells) 2. Type and Screen (60 min. TAT) 3. Cord Blood (2 hours) HEMATOLOGY 1. Complete Blood Count (CBC) SEE NOTE #1 2. Fibrinogen 3. Hemogram (ABC) 4. Monospot Test 5. Partial Thromboplastin Time (PTT) (60 min. TAT) 6. Prothrombin Time (PT) (60 min. TAT) 7. Fluid Cell Count (60 min. TAT) 8. Complete Urinalysis 9. D-dimer 10. Fluid pH CHEMISTRY 1. Arterial Blood Gases 2. Acetone 3. Ammonia 4. Amylase 5. Basic Metabolic Panel: Sodium, Potassium, Chloride, CO2, Creatinine, BUN, Glucose, and Calcium 6. Bilirubin (Total) 7. Blood Urea Nitrogen (BUN) 8. Calcium (Total and Ionized) 9. CPK (Total) (60 min. TAT for CPK total with MB fraction. CPKMB only run if CPK total > 113) UVM HEALTH NETWORK-CVPH 2 TEST MENU 10. Creatinine 11. CSF (Glucose and Protein) 12. Electrolytes: Sodium (Na), Potassium (K), Chloride (Cl), CO2 13. -

Effects of Hyperglycemia and Rapid Lowering of Plasma Glucose in Normal Rabbits
MILESTONES IN NEPHROLOGY J Am Soc Nephrol 11: 1776–1788, 2000 Mark A. Knepper, Feature Editor Studies on Mechanisms of Cerebral Edema in Diabetic Comas EFFECTS OF HYPERGLYCEMIA AND RAPID LOWERING OF PLASMA GLUCOSE IN NORMAL RABBITS ALLEN I. ARIEFF AND CHARLES R. KLEEMAN WITH THE TECHNICAL ASSISTANCE OF ALICE KEUSHKERIAN AND HELEN BAGDOYAN From the Departments of Medicine, Wadsworth Veterans Administration Center and Cedars-Sinai Medical Center, and the Cedars-Sinai Medical Research Institute, and University of California Los Angeles Medical Center, Los Angeles, California 90048 with comments by ALLEN I. ARIEFF AND RICHARD STERNS Reprinted from J. Clin. Invest. 52:571–583, 1973 A BSTRACT To investigate the pathophysiology of cerebral edema occurring during treatment of diabetic coma, the effects of hyperglycemia and rapid lowering of plasma glucose were AUTHOR COMMENTARY evaluated in normal rabbits. During 2 h of hyperglycemia (plasma glucose = 61 mM), both brain (cerebral cortex) and Allen I. Arieff muscle initially lost about 10% of water content. After 4 h of hyperglycemia, skeletal muscle water content remained low University of California, but that of brain was normal. Brain osmolality (Osm) (343 San Francisco mosmol/kg H2O) was similar to that of cerebrospinal fluid Sausalito, California (CSF) (340 mosmol/kg), but increases in the concentration of Na+, K+, Cl–, glucose, sorbitol, lactate, urea, myoinositol, and amino acids accounted for only about half of this increase. n 1936, Dillon, Riggs, and Dyer described a syndrome The unidentified solute was designated “idiogenic osmoles”. Iwhereby individuals who were being treated for diabetic When plasma glucose was rapidly lowered to normal with coma and apparently recovering suddenly deteriorated, with insulin, there was gross brain edema, increases in brain content worsening of coma, respiratory insufficiency, hypotension, of water, Na+, K+, Cl– and idiogenic osmoles, and a signifi tachycardia, high fever, and death (1). -

The Pathophysiology of 'Happy' Hypoxemia in COVID-19
Dhont et al. Respiratory Research (2020) 21:198 https://doi.org/10.1186/s12931-020-01462-5 REVIEW Open Access The pathophysiology of ‘happy’ hypoxemia in COVID-19 Sebastiaan Dhont1* , Eric Derom1,2, Eva Van Braeckel1,2, Pieter Depuydt1,3 and Bart N. Lambrecht1,2,4 Abstract The novel coronavirus disease 2019 (COVID-19) pandemic is a global crisis, challenging healthcare systems worldwide. Many patients present with a remarkable disconnect in rest between profound hypoxemia yet without proportional signs of respiratory distress (i.e. happy hypoxemia) and rapid deterioration can occur. This particular clinical presentation in COVID-19 patients contrasts with the experience of physicians usually treating critically ill patients in respiratory failure and ensuring timely referral to the intensive care unit can, therefore, be challenging. A thorough understanding of the pathophysiological determinants of respiratory drive and hypoxemia may promote a more complete comprehension of a patient’sclinical presentation and management. Preserved oxygen saturation despite low partial pressure of oxygen in arterial blood samples occur, due to leftward shift of the oxyhemoglobin dissociation curve induced by hypoxemia-driven hyperventilation as well as possible direct viral interactions with hemoglobin. Ventilation-perfusion mismatch, ranging from shunts to alveolar dead space ventilation, is the central hallmark and offers various therapeutic targets. Keywords: COVID-19, SARS-CoV-2, Respiratory failure, Hypoxemia, Dyspnea, Gas exchange Take home message COVID-19, little is known about its impact on lung This review describes the pathophysiological abnormal- pathophysiology. COVID-19 has a wide spectrum of ities in COVID-19 that might explain the disconnect be- clinical severity, data classifies cases as mild (81%), se- tween the severity of hypoxemia and the relatively mild vere (14%), or critical (5%) [1–3]. -

Tests…But Maybe You Transports Don’T 2
11/12/15 Some things you should When lab tests are useful know about laboratory 1. Managing patients during critical care tests…But maybe you transports don’t 2. While transporting patient to medical facilities for evaluation of laboratory Steve Faynor, CCEMT-P abnormalities HCA Chippenham Medical Center Richmond Ambulance Authority Objectives 1. Review some basic laboratory tests. Treat the patient, not the 2. Appreciate how patterns of laboratory test results can offer insight into etiology. laboratory values. 3. Learn how laboratory test calculations can add additional clinical information. 4. Review some limitations of laboratory tests. ELECTROLYTES & A case of “bad labs” RENAL FUNCTION TESTS 1 11/12/15 Hypernatremia & Renal Failure Hypernatremia • 89 year old white female • Hyperaldosteronism • Coming from nursing home due to • Cushing’s disease or syndrome abnormal labs • Diabetes insipidus (deficiency of ADH) • Sodium 172 mmol/L • Dehydration • Potassium 4.2 mmol/L • Chloride 137 mmol/L • Carbon dioxide 21 mmol/L • What are some causes of hypernatremia? • BP 122/66, SBP 99 later • BUN 212 mg N/dL • HR 64/min • Creatinine 6.10 mg/dL • RR 21/min • What do these values indicate? • SpCO2 98% on 4 L oxygen per min • Does this change your therapy? • Tongue dry, skin turgor poor • What is the cause of the hypernatremia in this patient? Treatment? Acute Renal Failure Use of the BUN/creatinine ratio • Intrinsic renal disease • In intrinsic causes of acute renal failure, the – Acute tubular necrosis: ischemia, toxins BUN/creatinine ratio is typically 10-15. – Acute glomerulonephritis • In pre-renal causes of acute renal failure, – CKD with missed dialysis the BUN/creatinine ratio is typically >20.