2021 Clinical Reference Ranges
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Evaluation of Abnormal Liver Chemistries
ACG Clinical Guideline: Evaluation of Abnormal Liver Chemistries Paul Y. Kwo, MD, FACG, FAASLD1, Stanley M. Cohen, MD, FACG, FAASLD2, and Joseph K. Lim, MD, FACG, FAASLD3 1Division of Gastroenterology/Hepatology, Department of Medicine, Stanford University School of Medicine, Palo Alto, California, USA; 2Digestive Health Institute, University Hospitals Cleveland Medical Center and Division of Gastroenterology and Liver Disease, Department of Medicine, Case Western Reserve University School of Medicine, Cleveland, Ohio, USA; 3Yale Viral Hepatitis Program, Yale University School of Medicine, New Haven, Connecticut, USA. Am J Gastroenterol 2017; 112:18–35; doi:10.1038/ajg.2016.517; published online 20 December 2016 Abstract Clinicians are required to assess abnormal liver chemistries on a daily basis. The most common liver chemistries ordered are serum alanine aminotransferase (ALT), aspartate aminotransferase (AST), alkaline phosphatase and bilirubin. These tests should be termed liver chemistries or liver tests. Hepatocellular injury is defined as disproportionate elevation of AST and ALT levels compared with alkaline phosphatase levels. Cholestatic injury is defined as disproportionate elevation of alkaline phosphatase level as compared with AST and ALT levels. The majority of bilirubin circulates as unconjugated bilirubin and an elevated conjugated bilirubin implies hepatocellular disease or cholestasis. Multiple studies have demonstrated that the presence of an elevated ALT has been associated with increased liver-related mortality. A true healthy normal ALT level ranges from 29 to 33 IU/l for males, 19 to 25 IU/l for females and levels above this should be assessed. The degree of elevation of ALT and or AST in the clinical setting helps guide the evaluation. -

Total Bilirubin in Athletes, Determination of Reference Range
OriginalTotal bilirubin Paper in athletes DOI: 10.5114/biolsport.2017.63732 Biol. Sport 2017;34:45-48 Total bilirubin in athletes, determination of reference range AUTHORS: Witek K1, Ścisłowska J2, Turowski D1, Lerczak K1, Lewandowska-Pachecka S2, Corresponding author: Pokrywka A3 Konrad Witek Department of Biochemistry, Institute of Sport - National 1 Department of Biochemistry, Institute of Sport - National Research Institute, Warsaw, Poland Research Institute 2 01-982 Warsaw, Poland Faculty of Pharmacy with the Laboratory Medicine Division, Medical University of Warsaw, Poland 3 E-mail: Department of Biochemistry, 2nd Faculty of Medicine, Medical University of Warsaw, Poland [email protected] ABSTRACT: The purpose of this study was to determine a typical reference range for the population of athletes. Results of blood tests of 339 athletes (82 women and 257 men, aged 18-37 years) were retrospectively analysed. The subjects were representatives of different sports disciplines. The measurements of total bilirubin (BIT), iron (Fe), alkaline phosphatase (ALP), alanine aminotransferase (ALT) and gamma-glutamyltransferase (GGT) were made using a Pentra 400 biochemical analyser (Horiba, France). Red blood cell count (RBC), reticulocyte count and haemoglobin concentration measurements were made using an Advia 120 haematology analyser (Siemens, Germany). In groups of women and men the percentage of elevated results were similar at 18%. Most results of total bilirubin in both sexes were in the range 7-14 μmol ∙ L-1 (49% of women and 42% of men). The highest results of elevated levels of BIT were in the range 21-28 μmol ∙ L-1 (12% of women and 11% of men). There was a significant correlation between serum iron and BIT concentration in female and male athletes whose serum total bilirubin concentration does not exceed the upper limit of the reference range. -

Normal Rangesа–А
Effective August 2007 5361 NW 33 rd Avenue Fort Lauderdale, FL 33309 954-777-0018 NORMAL RANGES – fax: 954-777-0211 ADULT MALE FEMALE CBC WBC 3.6 – 11.0 K/UL 3.6 – 11.0 RBC 4.5 – 5.9 M/UL 4.5 – 5.9 HEMOGLOBIN 13.0 – 18.0 G/DL 12.0 – 15.6 HEMATOCRIT 40 – 52 % 36.0 – 46.0 MCV 81 – 97 FL 81 93 MCH 26 – 34 PG 26 34 MCHC 31 – 37 gm/dl 31 37 RDW 11.5 – 15 % 11.5 – 15.0 PLATELET CT 150 – 400 K/UL 140 400 PLT VOL 7.4 – 10.4 FL 7.4 – 10.4 NEUTROPHIL % 36 – 66 36 75 LYMPHOCYTE % 23 – 43 27 47 MONOCYTE % 0.0 – 10 0.0 10 EOSINOPHIL % 0 – 5.0 0.0 – 5.0 BASOPHIL % 0 – 1.0 0.0 – 1.0 RETIC 0.4 – 1.9 0.4 – 1.9 COMPREHENSIVE METABOLIC PROFILE /LIVER PROFILE SODIUM 135 – 145 MMOL/L 135 145 POTASSIUM 3.5 – 5.5 MMOL/L 3.5 – 5.5 (OUTREACH) CHLORIDE 95 – 110 MMOL/L 95 110 CO2 19 – 34.MMOL/L 19 34 GLUCOSE 70 – 110 MG/DL 70 110 BUN 6 – 22 MG/DL 6 22 CREATININE 0.6 – 1.3 MG/DL 0.6 – 1.3 MG/DL CALCIUM 8.4 – 10.2 MG/DL 8.4 – 10.2 TOTAL PROTEIN 5.5 – 8.7 G/DL 5.5 – 8.7 ALBUMIN 3.2 – 5.0 g/dl 3.2 – 5.0 BILI TOTAL 0.1 – 1.2 MG/DL 0.1 – 1.2 BILI DIRECT 0.0 – 0.3 MG/DL 0.0 – 0.3 BILI INDIRECT 0.2 – 1.0 MG/DL 0.2 – 1.0 ALKALINE PHOS 20 – 130 U/L 20 130 AST/SGOT 10 – 40 U/L 10 40 ALT/SGPT 7 55 U/L 7 55 AST/ALT RATIO 0.5 – 0.9 RATIO 0.5 – 0.9 AMYLASE 25 – 115 U/L 25 – 115 U/L LIPASE 114 – 286 U/L 114 – 286 U/L CRP 0 – 0.9 MG/DL 0 – 0.9 MG/DL PREALBUMIN 18.0 – 35.7 mg/dL 18.0 – 35.7 mg/dL Revised 807 MAGNESIUM 1.8 – 2.4 mg/dL 1.8 – 2.4 mg/Dl LDH 100 – 200 Units /L 100 – 200 Units/L PHOSPHOROUS 2.3 – 5.0 mg/dL 2.3 – 5.0 mg/dL LACTIC ACID 0.4 -
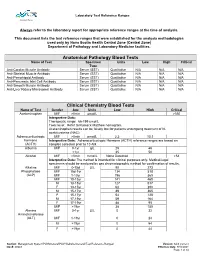
Laboratory Test Reference Ranges
Laboratory Test Reference Ranges Central Zone Always refer to the laboratory report for appropriate reference ranges at the time of analysis. This document lists the test reference ranges that were established for the analysis methodologies used only by Nova Scotia Health Central Zone (Central Zone) Department of Pathology and Laboratory Medicine facilities. Anatomical Pathology Blood Tests Name of Test Specimen Units Low High Critical Type Anti-Cardiac Muscle Antibody Serum (SST) Qualitative N/A N/A N/A Anti-Skeletal Muscle Antibody Serum (SST) Qualitative N/A N/A N/A Anti-Pemphigoid Antibody Serum (SST) Qualitative N/A N/A N/A Anti-Pancreatic Islet Cell Antibody Serum (SST) Qualitative N/A N/A N/A Anti-Smooth Muscle Antibody Serum (SST) Qualitative N/A N/A N/A Anti-Liver Kidney Microsomal Antibody Serum (SST) Qualitative N/A N/A N/A Clinical Chemistry Blood Tests Name of Test Gender Age Units Low High Critical Acetaminophen M/F >0min µmol/L >350 Interpretive Data: Therapeutic range: 66-199 umol/L Toxic level: Refer to Rumack Matthew nomogram. Acetaminophen results can be falsely low for patients undergoing treatment of N- acetylcysteine (NAC). Adrenocorticotropic M/F >0min pmol/L 2.3 10.1 hormone Interpretive Data: Adrenocorticotropic Hormone (ACTH) reference ranges are based on (ACTH) samples collected prior to 10 AM. Albumin M/F 0-1yr g/L 25 46 >1yr 35 50 Alcohol M/F >0min mmol/L None Detected >54 Interpretive Data: The method is intended for clinical purposes only. Medical-legal specimens should be analyzed by gas chromatographic method for confirmation of results. -

Cerebrospinal Fluid in Critical Illness
Cerebrospinal Fluid in Critical Illness B. VENKATESH, P. SCOTT, M. ZIEGENFUSS Intensive Care Facility, Division of Anaesthesiology and Intensive Care, Royal Brisbane Hospital, Brisbane, QUEENSLAND ABSTRACT Objective: To detail the physiology, pathophysiology and recent advances in diagnostic analysis of cerebrospinal fluid (CSF) in critical illness, and briefly review the pharmacokinetics and pharmaco- dynamics of drugs in the CSF when administered by the intravenous and intrathecal route. Data Sources: A review of articles published in peer reviewed journals from 1966 to 1999 and identified through a MEDLINE search on the cerebrospinal fluid. Summary of review: The examination of the CSF has become an integral part of the assessment of the critically ill neurological or neurosurgical patient. Its greatest value lies in the evaluation of meningitis. Recent publications describe the availability of new laboratory tests on the CSF in addition to the conventional cell count, protein sugar and microbiology studies. Whilst these additional tests have improved our understanding of the pathophysiology of the critically ill neurological/neurosurgical patient, they have a limited role in providing diagnostic or prognostic information. The literature pertaining to the use of these tests is reviewed together with a description of the alterations in CSF in critical illness. The pharmacokinetics and pharmacodynamics of drugs in the CSF, when administered by the intravenous and the intrathecal route, are also reviewed. Conclusions: The diagnostic utility of CSF investigation in critical illness is currently limited to the diagnosis of an infectious process. Studies that have demonstrated some usefulness of CSF analysis in predicting outcome in critical illness have not been able to show their superiority to conventional clinical examination. -

Determination of Reference Ranges for Selected Clinical Laboratory Tests for a Medical Laboratory in Namibia Using Pre-Tested Data
DETERMINATION OF REFERENCE RANGES FOR SELECTED CLINICAL LABORATORY TESTS FOR A MEDICAL LABORATORY IN NAMIBIA USING PRE-TESTED DATA by CORNELIA DE WAAL-MILLER Student no: 197084516 Thesis submitted in fulfillment of the requirements of the degree Master of Technology: Biomedical Technology in the Faculty of Health and Wellness Sciences at the Cape Peninsula University of Technology Supervisor: Professor A.J. Esterhuyse Co-Supervisor: Professor B. Noden Bellville March 2015 CPUT copyright information The thesis may not be published either in part (in scholarly, scientific or technical journals), or as a whole (as a monograph), unless permission has been obtained from the University. (i) Declaration I, Cornelia de Waal-Miller, declare that the content of this thesis represents my own unaided work, and that this thesis has not previously been submitted for academic examination towards any qualification. Furthermore, this thesis represents my own opinions and not necessarily those of the Cape Peninsula University of Technology. 12th March 2015 Signed Date 2 | of 84 Pages (ii) Abstract Aim: The aim of the study was to compile pre-tested laboratory results stored in the laboratory database of the Namibia Institute of Pathology (NIP). The study also aimed to assess the usefulness and validity of using retrospective laboratory results of different patients in varying degrees of health and which were produced using various methods in different laboratories in Namibia. Methods: 254,271 test results (female: 134,261, male = 117,091, unknown gender= 2,919) consisting of Haemoglobin, serum Urea, serum Creatinine, plasma Glucose (fasting and random), serum Cholesterol, serum Triglycerides and serum Uric Acid was extracted from NIP Laboratory Information System over a period of four years and of the 13 different regions of Namibia were analyzed. -

CSF Xanthochromia
CSF Xanthochromia Pseudonyms – CSF bilirubin For investigation of suspected subarachnoid haemorrhage (SAH) in CT negative patients Xanthochromia is the yellow discoloration indicating the presence of bilirubin in CSF which appears as oxyhaemoglobin released from the breakdown of red blood cells following haemorrhage into the CSF is converted in vivo into bilirubin in a time‐dependent manner. A subarachnoid haemorrhage (SAH) is a spontaneous arterial bleeding into the subarachnoid space, usually from a cerebral aneurysm, and characterised by a severe sudden‐onset headache. The majority of positive cases are detected by computed tomography (CT) scanning but for those CT‐ negative patients presenting with a history suggestive of SAH the measurement of xanthochromia in CSF is advocated to detect those patients who actually have sustained a SAH and require treatment and to eliminate the possibility of SAH in the remainder without the need of confirmatory angiography. The CSF is collected by means of lumbar puncture (LP). For full interpretation of the result other CSF and blood tests must be collected at the same time – CSF protein and glucose; plasma glucose; and serum protein and bilirubin. General information CSF Xanthochromia sample collection kits are provided in the ACU (ORC) and AMU (Trafford) and also available from the Specimen Receptions at the laboratories at both sites. Concurrent samples should be requested for CSF protein and glucose, plasma glucose and serum LFTS (for protein and bilirubin) using the sample containers supplied. Collection container: CSF xanthochromia: White topped Universal container (In addition the following samples should be collected:‐ CSF glucose & protein: 1.2 mL fluoride‐EDTA glucose (Sarstedt yellow top) Serum protein & bilirubin: 4.9 mL SST (Sarstedt brown top) Plasma glucose: 2.7 mL fluoride‐EDTA glucose (Sarstedt yellow top) Type and volume of sample: 1 mL CSF requested, minimum 400 µL required for analysis Specimen transport/special precautions: A CSF Xanthochromia sample collection kit should be used. -
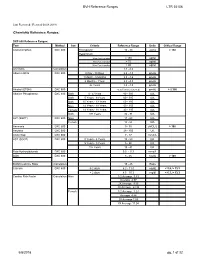
BVH Reference Ranges LTR 35106 Chemistry Reference Ranges
BVH Reference Ranges LTR 35106 Last Reviewed: (Revised 05.08.2018) Chemistry Reference Ranges: DXC 600 Reference Ranges: Test Method Sex Criteria Reference Range Units Critical Range Acetaminophen DXC 600 Therapeutic 10 - 30 ug/mL > 150 Hepatotoxic 4 hrs Post Ingestion > 150 ug/ml 8 hrs Post Ingestion > 75 ug/ml 12 hrs Post Ingestion > 40 ug/ml A/G Ratio Calculation 1.1 - 2.2 Albumin BCG DXC 600 0 Day - 30 Days 2.6 - 4.3 gm/dL 1 Month - 5 Months 2.8 - 4.6 gm/dL 6 Months - 1Year 2.8 - 4.8 gm/dL 2+ Years 3.2 - 4.9 gm/dL Alcohol (ETOH) DXC 600 <0.005 (none detected) gm/dL > 0.300 Alkaline Phosphatase DXC 600 Both 0 - 4 Years 80 - 350 IU/L Both 5 Years - 9 Years 60 - 385 IU/L Both 10 Years - 13 Years 60 - 485 IU/L Male 14 Years - 18 Years 50 - 350 IU/L Female 14 Years - 18 Years 40 - 195 IU/L Both 19+ Years 32 - 91 IU/L ALT (SGPT) DXC 600 Male 17 - 63 IU/L Female 14 - 54 IU/L Ammonia DXC 600 9 - 35 uMOL/L > 100 Amylase DXC 600 28 - 100 U/L Anion Gap DXC 600 7 - 17 mmol/L AST (SGOT) DXC 600 0 Years - 4 Years 10 - 60 IU/L 5 Years - 9 Years 5 - 50 IU/L 10+ Years 15 - 41 IU/L Beta Hydroxybutyrate DXC 600 0.0 - 0.3 mmol/L BUN DXC 600 8 - 26 mg/dL > 100 BUN/Creatinine Ratio Calculation 15 - 25 Ratio Calcium DXC 600 0-2 days 6.2 - 11.0 mg/dL < 5.8, > 13.3 > 2 days 8.5 - 10.3 mg/dl < 6.3, > 13.3 Cardiac Risk Factor Calculation Male 1/2 Average 3.43 Average 4.97 2X Average 9.55 3X Average 23.99 Female 1/2 Average 3.27 Average 4.44 2X Average 7.05 3X Average 11.04 5/8/2018 pg. -

Routine Cerebrospinal Fluid (CSF) Analysis
CHAPTER4 Routine cerebrospinal fluid (CSF) analysis a b a ) F. Deisenhammer, A. Bartos, R. Egg, Albumin CSF/serum ratio (Qalb should be pre- N. E. Gilhus,c G. Giovannoni,d S. Rauer,e ferred to total protein measurement and normal F. Sellebjergf upper limits should be related to patients’ age. Elevated Qalb is a non-specific finding but occurs mainly in bacterial, cryptococcal, and tubercu- Background A great variety of neurological diseases lous meningitis, leptomingeal metastases as well require investigation of the cerebrospinal fluid as acute and chronic demyelinating polyneu- (CSF) to prove the diagnosis or to rule out relevant ropathies. differential diagnoses. Pathological decrease of the CSF/serum glu- cose ratio or an increase in lactate concentration Objectives To evaluate the theoretical background indicates bacterial or fungal meningitis or lep- and provide guidelines for clinical use in rou- tomeningeal metastases. tine CSF analysis including total protein, albumin, Intrathecal immunoglobulin G synthesis is best immunoglobulins, glucose, lactate, cell count, demonstrated by isoelectric focusing followed by cytological staining, and investigation of infec- specific staining. tious CSF. Cellular morphology (cytological staining) should be evaluated whenever pleocytosis is found or Methods Systematic Medline search for the above leptomeningeal metastases or pathological bleed- mentioned variables. Review of appropriate publi- ing is suspected. Computed tomography-negative cations by one or more of the task force members. intrathecal bleeding should be investigated by Grading of evidence and recommendations was bilirubin detection. based on consensus by all task force members. Introduction CSF should be analysed immediately after collec- tion. If storage is needed 12 ml of CSF should The cerebrospinal fluid (CSF) is a dynamic, be partitioned into three to four sterile tubes. -

Calcium, Plasma, Total
Division of Laboratory Medicine Biochemistry Calcium, plasma, total Calcium is the most abundant mineral element in the body with about 99 percent in the bones primarily as hydroxyapatite. The remaining calcium is distributed between the various tissues and the extracellular fluids where it performs a vital role for many life sustaining processes. Among the extra skeletal functions of calcium are involvement in blood coagulation, neuromuscular conduction, excitability of skeletal and cardiac muscle, enzyme activation, and the preservation of cell membrane integrity and permeability. Calcium levels and hence the body content are controlled by parathyroid hormone (PTH), calcitonin, and vitamin D. To maintain calcium homeostasis, we require sufficient intake, normal metabolism and appropriate excretion. An imbalance in any of these modulators leads to alterations of the body and circulating calcium levels. Increases in serum PTH or vitamin D are usually associated with hypercalcemia. Increased calcium levels may also be observed in multiple myeloma and other neoplastic diseases. Hypocalcemia may be observed e.g. in hypoparathyroidism, nephrosis, and pancreatitis. Total Calcium is reported as direct and after adjustment for plasma albumin level. Adjusted total calcium has the same reference interval as total calcium and is a surrogate for ionised calcium. General information Collection container: Adults – serum (with gel separator, 4.9mL brown top Sarstedt tube) Paediatrics – lithium heparin plasma (1.2mL orange top Sarstedt tube) Type and volume of sample: The tubes should be thoroughly mixed before transport to the lab. 1mL whole blood is required as a minimum volume. Specimen transport/special precautions: N/A Laboratory information Method principle: Calcium ions react with 5‑nitro‑5’‑methyl‑BAPTA (NM‑BAPTA) under alkaline conditions to form a complex. -

Lab Dept: Chemistry Test Name: VENOUS BLOOD GAS (VBG)
Lab Dept: Chemistry Test Name: VENOUS BLOOD GAS (VBG) General Information Lab Order Codes: VBG Synonyms: Venous blood gas CPT Codes: 82803 - Gases, blood, any combination of pH, pCO2, pO2, CO2, HCO3 (including calculated O2 saturation) Test Includes: VpH (no units), VpCO2 and VpO2 measured in mmHg, VsO2 and VO2AD measured in %, HCO3 and BE measured in mmol/L, Temperature (degrees C) and ST (specimen type) Logistics Test Indications: Useful for evaluating oxygen and carbon dioxide gas exchange; respiratory function, including hypoxia; and acid/base balance. It is also useful in assessment of asthma; chronic obstructive pulmonary disease and other types of lung disease; embolism, including fat embolism; and coronary artery disease. Lab Testing Sections: Chemistry Phone Numbers: MIN Lab: 612-813-6280 STP Lab: 651-220-6550 Test Availability: Daily, 24 hours Turnaround Time: 30 minutes Special Instructions: See Collection and Patient Preparation Specimen Specimen Type: Whole blood Container: Preferred: Sims Portex® syringe (PB151) or Smooth-E syringe (956- 463) Draw Volume: 0.4 mL (Minimum: 0.2 mL) blood Note: Submission of 0.2 mL of blood does not allow for repeat analysis. Processed Volume: 0.2 mL blood per analysis Collection: Avoid using a tourniquet. Anaerobically collect blood into a heparinized blood gas syringe (See Container. Once the puncture has been performed or the line specimen drawn, immediately remove all air from the syringe. Remove the needle, cap tightly and gently mix. Do not expose the specimen to air. Forward the specimen immediately at ambient temperature. Specimens cannot be stored. Note: When drawing from an indwelling catheter, the line must be thoroughly flushed with blood before drawing the sample. -
D40. Cerebrospinal Fluid.Pdf
CEREBROSPINAL FLUID D40 (1) Cerebrospinal Fluid (CSF) Last updated: June 3, 2019 PHYSIOLOGY ............................................................................................................................................ 1 CSF PRODUCTION .................................................................................................................................. 1 CSF REABSORPTION ............................................................................................................................... 1 PARAMETERS ........................................................................................................................................... 2 NORMAL ................................................................................................................................................ 2 OPENING PRESSURE ................................................................................................................................ 3 COLOR ................................................................................................................................................... 3 BLOODY CSF ......................................................................................................................................... 3 VISCOSITY & TURBIDITY ....................................................................................................................... 4 CELLS ...................................................................................................................................................