Enhance Detection of Recent Hyperglycemia with a Simple Blood Test
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Management of Diabetes Mellitus Standards of Care and Clinical Practice Guidelines
WHO-EM/DIN6/E/G MANAGEMENT OF DIABETES MELLITUS STANDARDS OF CARE AND CLINICAL PRACTICE GUIDELINES Edited by Dr A.A.S. Alwan Regional Adviser, Noncommunicable Diseases WHO Regional Office for the Eastern Mediterranean WHO-EM/DIN6/E/G INTRODUCTION Available data from many countries of the Eastern Mediterranean Region (EMR) indicate that diabetes mellitus has become a problem of great magnitude and a major public health concern. Studies have demonstrated that, in some countries, diabetes affects up to 10% of the population aged 20 years and older. This rate may be doubled if those with impaired glucose tolerance (IGT) are also included. The manifestations of diabetes cause considerable human suffering and enormous economic costs. Both acute and late diabetic complications are commonly encountered. Long-term complications represented by cardiovascular diseases, cerebrovascular accidents, end-stage renal disease, retinopathy and neuropathies are already major causes of morbidity, disability and premature death in countries of this Region. The development of long-term complications is influenced by hyperglycaernia. Poor control of diabetes accelerates their progression. Thus, to prevent complications, good control of diabetes is essential and the management of diabetes should therefore aim to improve glycaemic control beyond that required to control its symptoms. Intensified therapy and maintaining near-normal blood glucose levels can result in considerable reduction in the risk of development of retinopathy, nephropathy and neuropathy. However, despite the high prevalence of diabetes and its complications and the availability of successful prevention strategies, essential health care requirements and facilities for self-care are often inadequate in this Region. Action is needed at all levels of health care and in the various aspects of diabetes care to bridge this gap and to improve health care delivery to people with diabetes. -

Evaluation of Abnormal Liver Chemistries
ACG Clinical Guideline: Evaluation of Abnormal Liver Chemistries Paul Y. Kwo, MD, FACG, FAASLD1, Stanley M. Cohen, MD, FACG, FAASLD2, and Joseph K. Lim, MD, FACG, FAASLD3 1Division of Gastroenterology/Hepatology, Department of Medicine, Stanford University School of Medicine, Palo Alto, California, USA; 2Digestive Health Institute, University Hospitals Cleveland Medical Center and Division of Gastroenterology and Liver Disease, Department of Medicine, Case Western Reserve University School of Medicine, Cleveland, Ohio, USA; 3Yale Viral Hepatitis Program, Yale University School of Medicine, New Haven, Connecticut, USA. Am J Gastroenterol 2017; 112:18–35; doi:10.1038/ajg.2016.517; published online 20 December 2016 Abstract Clinicians are required to assess abnormal liver chemistries on a daily basis. The most common liver chemistries ordered are serum alanine aminotransferase (ALT), aspartate aminotransferase (AST), alkaline phosphatase and bilirubin. These tests should be termed liver chemistries or liver tests. Hepatocellular injury is defined as disproportionate elevation of AST and ALT levels compared with alkaline phosphatase levels. Cholestatic injury is defined as disproportionate elevation of alkaline phosphatase level as compared with AST and ALT levels. The majority of bilirubin circulates as unconjugated bilirubin and an elevated conjugated bilirubin implies hepatocellular disease or cholestasis. Multiple studies have demonstrated that the presence of an elevated ALT has been associated with increased liver-related mortality. A true healthy normal ALT level ranges from 29 to 33 IU/l for males, 19 to 25 IU/l for females and levels above this should be assessed. The degree of elevation of ALT and or AST in the clinical setting helps guide the evaluation. -

Total Bilirubin in Athletes, Determination of Reference Range
OriginalTotal bilirubin Paper in athletes DOI: 10.5114/biolsport.2017.63732 Biol. Sport 2017;34:45-48 Total bilirubin in athletes, determination of reference range AUTHORS: Witek K1, Ścisłowska J2, Turowski D1, Lerczak K1, Lewandowska-Pachecka S2, Corresponding author: Pokrywka A3 Konrad Witek Department of Biochemistry, Institute of Sport - National 1 Department of Biochemistry, Institute of Sport - National Research Institute, Warsaw, Poland Research Institute 2 01-982 Warsaw, Poland Faculty of Pharmacy with the Laboratory Medicine Division, Medical University of Warsaw, Poland 3 E-mail: Department of Biochemistry, 2nd Faculty of Medicine, Medical University of Warsaw, Poland [email protected] ABSTRACT: The purpose of this study was to determine a typical reference range for the population of athletes. Results of blood tests of 339 athletes (82 women and 257 men, aged 18-37 years) were retrospectively analysed. The subjects were representatives of different sports disciplines. The measurements of total bilirubin (BIT), iron (Fe), alkaline phosphatase (ALP), alanine aminotransferase (ALT) and gamma-glutamyltransferase (GGT) were made using a Pentra 400 biochemical analyser (Horiba, France). Red blood cell count (RBC), reticulocyte count and haemoglobin concentration measurements were made using an Advia 120 haematology analyser (Siemens, Germany). In groups of women and men the percentage of elevated results were similar at 18%. Most results of total bilirubin in both sexes were in the range 7-14 μmol ∙ L-1 (49% of women and 42% of men). The highest results of elevated levels of BIT were in the range 21-28 μmol ∙ L-1 (12% of women and 11% of men). There was a significant correlation between serum iron and BIT concentration in female and male athletes whose serum total bilirubin concentration does not exceed the upper limit of the reference range. -

Normal Rangesа–А
Effective August 2007 5361 NW 33 rd Avenue Fort Lauderdale, FL 33309 954-777-0018 NORMAL RANGES – fax: 954-777-0211 ADULT MALE FEMALE CBC WBC 3.6 – 11.0 K/UL 3.6 – 11.0 RBC 4.5 – 5.9 M/UL 4.5 – 5.9 HEMOGLOBIN 13.0 – 18.0 G/DL 12.0 – 15.6 HEMATOCRIT 40 – 52 % 36.0 – 46.0 MCV 81 – 97 FL 81 93 MCH 26 – 34 PG 26 34 MCHC 31 – 37 gm/dl 31 37 RDW 11.5 – 15 % 11.5 – 15.0 PLATELET CT 150 – 400 K/UL 140 400 PLT VOL 7.4 – 10.4 FL 7.4 – 10.4 NEUTROPHIL % 36 – 66 36 75 LYMPHOCYTE % 23 – 43 27 47 MONOCYTE % 0.0 – 10 0.0 10 EOSINOPHIL % 0 – 5.0 0.0 – 5.0 BASOPHIL % 0 – 1.0 0.0 – 1.0 RETIC 0.4 – 1.9 0.4 – 1.9 COMPREHENSIVE METABOLIC PROFILE /LIVER PROFILE SODIUM 135 – 145 MMOL/L 135 145 POTASSIUM 3.5 – 5.5 MMOL/L 3.5 – 5.5 (OUTREACH) CHLORIDE 95 – 110 MMOL/L 95 110 CO2 19 – 34.MMOL/L 19 34 GLUCOSE 70 – 110 MG/DL 70 110 BUN 6 – 22 MG/DL 6 22 CREATININE 0.6 – 1.3 MG/DL 0.6 – 1.3 MG/DL CALCIUM 8.4 – 10.2 MG/DL 8.4 – 10.2 TOTAL PROTEIN 5.5 – 8.7 G/DL 5.5 – 8.7 ALBUMIN 3.2 – 5.0 g/dl 3.2 – 5.0 BILI TOTAL 0.1 – 1.2 MG/DL 0.1 – 1.2 BILI DIRECT 0.0 – 0.3 MG/DL 0.0 – 0.3 BILI INDIRECT 0.2 – 1.0 MG/DL 0.2 – 1.0 ALKALINE PHOS 20 – 130 U/L 20 130 AST/SGOT 10 – 40 U/L 10 40 ALT/SGPT 7 55 U/L 7 55 AST/ALT RATIO 0.5 – 0.9 RATIO 0.5 – 0.9 AMYLASE 25 – 115 U/L 25 – 115 U/L LIPASE 114 – 286 U/L 114 – 286 U/L CRP 0 – 0.9 MG/DL 0 – 0.9 MG/DL PREALBUMIN 18.0 – 35.7 mg/dL 18.0 – 35.7 mg/dL Revised 807 MAGNESIUM 1.8 – 2.4 mg/dL 1.8 – 2.4 mg/Dl LDH 100 – 200 Units /L 100 – 200 Units/L PHOSPHOROUS 2.3 – 5.0 mg/dL 2.3 – 5.0 mg/dL LACTIC ACID 0.4 -

Clinical Use of Hemoglobin A1c to Improve Diabetes Management
PRACTICAL POINTERS Clinical Use of Hemoglobin A1c to Improve Diabetes Management Alan M. Delamater, PhD, ABPP or more than 25 years, the hemo- one recent study conducted in Norway6 A1C values. Only 14% of the youths globin A1c (A1C) test has been revealed that the majority (82.6%) of were able to accurately describe the A1C Fthe most widely accepted out- 201 adult patients with type 1 diabetes test. Just 11, 7.8, and 7.8% correctly come measure for evaluating glycemic knew what their last A1C was, and most identified the A1C ranges for good, fair, control in individuals with diabetes. patients (90%) knew what a satisfactory and poor glycemic control, respectively. The test provides an index of a patient’s A1C value would be. But a significant Very few youths (1.6–3.2%) knew the average blood glucose level during the number of patients (42%) reported they blood glucose values corresponding to past 2–3 months1 and is considered to had low knowledge of A1C testing in specific A1C results. Only a small num- be the most objective and reliable general. Furthermore, 25% of patients ber of youths correctly estimated the measure of long-term metabolic con- did not think that treatment intensifica- short- and long-term risks associated trol.2,3 The Diabetes Control and tion should occur at an A1C value of with A1C values of 7 and 12%. In this Complications Trial established that 10%. sample, there was a significant lack of maintaining A1C levels as close as pos- A recent cross-sectional study knowledge concerning the meaning and sible to the normal range results in con- examined the relationship between implications of the A1C test. -
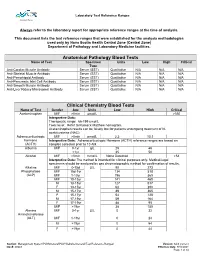
Laboratory Test Reference Ranges
Laboratory Test Reference Ranges Central Zone Always refer to the laboratory report for appropriate reference ranges at the time of analysis. This document lists the test reference ranges that were established for the analysis methodologies used only by Nova Scotia Health Central Zone (Central Zone) Department of Pathology and Laboratory Medicine facilities. Anatomical Pathology Blood Tests Name of Test Specimen Units Low High Critical Type Anti-Cardiac Muscle Antibody Serum (SST) Qualitative N/A N/A N/A Anti-Skeletal Muscle Antibody Serum (SST) Qualitative N/A N/A N/A Anti-Pemphigoid Antibody Serum (SST) Qualitative N/A N/A N/A Anti-Pancreatic Islet Cell Antibody Serum (SST) Qualitative N/A N/A N/A Anti-Smooth Muscle Antibody Serum (SST) Qualitative N/A N/A N/A Anti-Liver Kidney Microsomal Antibody Serum (SST) Qualitative N/A N/A N/A Clinical Chemistry Blood Tests Name of Test Gender Age Units Low High Critical Acetaminophen M/F >0min µmol/L >350 Interpretive Data: Therapeutic range: 66-199 umol/L Toxic level: Refer to Rumack Matthew nomogram. Acetaminophen results can be falsely low for patients undergoing treatment of N- acetylcysteine (NAC). Adrenocorticotropic M/F >0min pmol/L 2.3 10.1 hormone Interpretive Data: Adrenocorticotropic Hormone (ACTH) reference ranges are based on (ACTH) samples collected prior to 10 AM. Albumin M/F 0-1yr g/L 25 46 >1yr 35 50 Alcohol M/F >0min mmol/L None Detected >54 Interpretive Data: The method is intended for clinical purposes only. Medical-legal specimens should be analyzed by gas chromatographic method for confirmation of results. -

Determination of Reference Ranges for Selected Clinical Laboratory Tests for a Medical Laboratory in Namibia Using Pre-Tested Data
DETERMINATION OF REFERENCE RANGES FOR SELECTED CLINICAL LABORATORY TESTS FOR A MEDICAL LABORATORY IN NAMIBIA USING PRE-TESTED DATA by CORNELIA DE WAAL-MILLER Student no: 197084516 Thesis submitted in fulfillment of the requirements of the degree Master of Technology: Biomedical Technology in the Faculty of Health and Wellness Sciences at the Cape Peninsula University of Technology Supervisor: Professor A.J. Esterhuyse Co-Supervisor: Professor B. Noden Bellville March 2015 CPUT copyright information The thesis may not be published either in part (in scholarly, scientific or technical journals), or as a whole (as a monograph), unless permission has been obtained from the University. (i) Declaration I, Cornelia de Waal-Miller, declare that the content of this thesis represents my own unaided work, and that this thesis has not previously been submitted for academic examination towards any qualification. Furthermore, this thesis represents my own opinions and not necessarily those of the Cape Peninsula University of Technology. 12th March 2015 Signed Date 2 | of 84 Pages (ii) Abstract Aim: The aim of the study was to compile pre-tested laboratory results stored in the laboratory database of the Namibia Institute of Pathology (NIP). The study also aimed to assess the usefulness and validity of using retrospective laboratory results of different patients in varying degrees of health and which were produced using various methods in different laboratories in Namibia. Methods: 254,271 test results (female: 134,261, male = 117,091, unknown gender= 2,919) consisting of Haemoglobin, serum Urea, serum Creatinine, plasma Glucose (fasting and random), serum Cholesterol, serum Triglycerides and serum Uric Acid was extracted from NIP Laboratory Information System over a period of four years and of the 13 different regions of Namibia were analyzed. -

Glossary of Technical Terms
THIS DOCUMENT IS IN DRAFT FORM, INCOMPLETE AND SUBJECT TO CHANGE AND THAT THE INFORMATION MUST BE READ IN CONJUNCTION WITH THE SECTION HEADED “WARNING” ON THE COVER OF THIS DOCUMENT. GLOSSARY OF TECHNICAL TERMS This glossary contains explanations of certain technical terms used in this Document in connection with our Company and its business. Such terminology and meanings may not correspond to standard industry meanings or usages of those terms. “acetaminophen” a non-opioid analgesic and antipyretic agent used to treat pain and fever “artificial pancreas” an integrated diabetes management system that tracks blood glucose levels using a continuous glucose monitor and automatically delivers the insulin when needed using an insulin pump according to its control algorithm “ascorbic acid” a potent reducing and antioxidant agent that functions in fighting bacterial infections, in detoxifying reactions, and in the formation of collagen in fibrous tissue, teeth, bones, connective tissue, skin, and capillaries “basal insulin” a small, continuous infusion of background insulin delivered automatically at a programmed rate, all day and night “BG Port” blood glucose strip port, the port that accepts and electrically connects a disposable blood glucose strip to the electronics “BGMS” blood glucose monitoring system “BLE” bluetooth low energy “blood glucose” blood glucose, also referred to as blood sugar, is the amount of glucose in your blood, an indicator of diabetes monitoring “bolus insulin” insulin that is taken to lower abnormally high blood glucose -

Calcium, Plasma, Total
Division of Laboratory Medicine Biochemistry Calcium, plasma, total Calcium is the most abundant mineral element in the body with about 99 percent in the bones primarily as hydroxyapatite. The remaining calcium is distributed between the various tissues and the extracellular fluids where it performs a vital role for many life sustaining processes. Among the extra skeletal functions of calcium are involvement in blood coagulation, neuromuscular conduction, excitability of skeletal and cardiac muscle, enzyme activation, and the preservation of cell membrane integrity and permeability. Calcium levels and hence the body content are controlled by parathyroid hormone (PTH), calcitonin, and vitamin D. To maintain calcium homeostasis, we require sufficient intake, normal metabolism and appropriate excretion. An imbalance in any of these modulators leads to alterations of the body and circulating calcium levels. Increases in serum PTH or vitamin D are usually associated with hypercalcemia. Increased calcium levels may also be observed in multiple myeloma and other neoplastic diseases. Hypocalcemia may be observed e.g. in hypoparathyroidism, nephrosis, and pancreatitis. Total Calcium is reported as direct and after adjustment for plasma albumin level. Adjusted total calcium has the same reference interval as total calcium and is a surrogate for ionised calcium. General information Collection container: Adults – serum (with gel separator, 4.9mL brown top Sarstedt tube) Paediatrics – lithium heparin plasma (1.2mL orange top Sarstedt tube) Type and volume of sample: The tubes should be thoroughly mixed before transport to the lab. 1mL whole blood is required as a minimum volume. Specimen transport/special precautions: N/A Laboratory information Method principle: Calcium ions react with 5‑nitro‑5’‑methyl‑BAPTA (NM‑BAPTA) under alkaline conditions to form a complex. -

Lab Dept: Chemistry Test Name: VENOUS BLOOD GAS (VBG)
Lab Dept: Chemistry Test Name: VENOUS BLOOD GAS (VBG) General Information Lab Order Codes: VBG Synonyms: Venous blood gas CPT Codes: 82803 - Gases, blood, any combination of pH, pCO2, pO2, CO2, HCO3 (including calculated O2 saturation) Test Includes: VpH (no units), VpCO2 and VpO2 measured in mmHg, VsO2 and VO2AD measured in %, HCO3 and BE measured in mmol/L, Temperature (degrees C) and ST (specimen type) Logistics Test Indications: Useful for evaluating oxygen and carbon dioxide gas exchange; respiratory function, including hypoxia; and acid/base balance. It is also useful in assessment of asthma; chronic obstructive pulmonary disease and other types of lung disease; embolism, including fat embolism; and coronary artery disease. Lab Testing Sections: Chemistry Phone Numbers: MIN Lab: 612-813-6280 STP Lab: 651-220-6550 Test Availability: Daily, 24 hours Turnaround Time: 30 minutes Special Instructions: See Collection and Patient Preparation Specimen Specimen Type: Whole blood Container: Preferred: Sims Portex® syringe (PB151) or Smooth-E syringe (956- 463) Draw Volume: 0.4 mL (Minimum: 0.2 mL) blood Note: Submission of 0.2 mL of blood does not allow for repeat analysis. Processed Volume: 0.2 mL blood per analysis Collection: Avoid using a tourniquet. Anaerobically collect blood into a heparinized blood gas syringe (See Container. Once the puncture has been performed or the line specimen drawn, immediately remove all air from the syringe. Remove the needle, cap tightly and gently mix. Do not expose the specimen to air. Forward the specimen immediately at ambient temperature. Specimens cannot be stored. Note: When drawing from an indwelling catheter, the line must be thoroughly flushed with blood before drawing the sample. -

Diabetes Management: Directory of Provider Resources (PDF)
DIABETES MANAGEMENT: DIRECTORY OF PROVIDER RESOURCES 2 Diabetes Management: Directory of Provider Resources ACKNOWLEDGMENTS Diabetes Management: Directory of Provider Resources was prepared with input from National Institutes of Health National Institute of Diabetes and Digestive and Kidney Diseases; Centers for Medicare & Medicaid Services Advisory Panel on Outreach and Education; NORC at the University of Chicago; Jamie Murkey, MPH, PhD, Program Alignment and Partner Engagement Group 2019 summer intern; Asian Services in Action – International Community Health Center; Chinatown Public Health Center; Colorado Coalition for the Homeless; Garden City Community Health Center, Genesis Family Health; Jackson Medical Mall and Jackson Hinds Comprehensive Health Center; Montefiore Medical Center; Nash Health Care Systems; Sun Life Family Health Center; and Bryan W. Whitfield Memorial Hospital, Tombigbee Healthcare Authority. 3 Diabetes Management: Directory of Provider Resources PURPOSE The purpose of this directory is to support providers and care teams by identifying resources on the management of type 2 diabetes. It is particularly suited for providers who work with Medicare beneficiaries and vulnerable populations for whom the prevalence of type 2 diabetes and diabetes complications is higher. This directory will help the care team identify resources to improve diabetes management by promoting medication adherence. This directory also aims to equip primary care teams with tools to manage diabetes and that patients with more complex needs are appropriately referred to specialists. While some patients require care from endocrinologists, primary care teams can effectively manage many patients with prediabetes and type 2 diabetes.i Other health professionals and patients can play an important role in facilitating medication management and other diabetes self-care behaviors. -

Uric Acid 7D76-20 30-3928/R4
URIC ACID 7D76-20 30-3928/R4 URIC ACID This package insert contains information to run the Uric Acid assay on the ARCHITECT c Systems™ and the AEROSET System. NOTE: Changes Highlighted NOTE: This package insert must be read carefully prior to product use. Package insert instructions must be followed accordingly. Reliability of assay results cannot be guaranteed if there are any deviations from the instructions in this package insert. Customer Support United States: 1-877-4ABBOTT Canada: 1-800-387-8378 (English speaking customers) 1-800-465-2675 (French speaking customers) International: Call your local Abbott representative Symbols in Product Labeling Calibrators 1 and 2 Catalog number/List number Concentration Serial number Authorized Representative in the Consult instructions for use European Community Ingredients Manufacturer In vitro diagnostic medical device Temperature limitation Batch code/Lot number Use by/Expiration date Reagent 1 ABBOTT LABORATORIES ABBOTT Abbott Park, IL 60064, USA Max-Planck-Ring 2 65205 Wiesbaden Germany +49-6122-580 July 2007 ©2002, 2007 Abbott Laboratories 1 NAME SPECIMEN COLLECTION AND HANDLING URIC ACID Suitable Specimens Serum, plasma, and urine are acceptable specimens. INTENDED USE • Serum: Use serum collected by standard venipuncture techniques The Uric Acid assay is used for the quantitation of uric acid in human into glass or plastic tubes with or without gel barriers. Ensure serum, plasma, or urine. complete clot formation has taken place prior to centrifugation. When processing samples, separate serum from blood cells or SUMMARY AND EXPLANATION OF TEST gel according to the specimen collection tube manufacturer’s Uric acid is a metabolite of purines, nucleic acids, and nucleoproteins.