Magnificent Magpie Colours by Feathers with Layers of Hollow Melanosomes Doekele G
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

BSTE744 2020.Pdf
Science of the Total Environment 744 (2020) 140895 Contents lists available at ScienceDirect Science of the Total Environment journal homepage: www.elsevier.com/locate/scitotenv Revisiting an old question: Which predators eat eggs of ground-nesting birds in farmland landscapes? Carolina Bravo a,b,⁎, Olivier Pays b,c, Mathieu Sarasa d,e, Vincent Bretagnolle a,f a Centre d'Etudes Biologiques de Chizé, UMR 7372, CNRS and La Rochelle Université, F-79360 Beauvoir-sur- Niort, France b LETG-Angers, UMR 6554, CNRS, Université d'Angers, 49045 Angers, France c REHABS International Research Laboratory, CNRS-Université Lyon 1-Nelson Mandela University, George Campus, Madiba drive, 6531 George, South Africa d BEOPS, 1 Esplanade Compans Caffarelli, 31000 Toulouse, France e Fédération Nationale des Chasseurs, 92136 Issy-les-Moulineaux cedex, France f LTSER “Zone Atelier Plaine & Val de Sèvre”, CNRS, 79360 Villiers-en-Bois, France HIGHLIGHTS GRAPHICAL ABSTRACT • Predation probability in artificial nests decreased with camera trap presence. • Corvids might perceive differently plas- ticine and natural eggs. • Camera trap and plasticine eggs combi- nation are recommendable for identify- ing predator. • Corvid predation increased with the abundance of corvid breeders. • Considering social status of corvids is es- sential when assessing corvid abun- dance impact. article info abstract Article history: Nest predation is a major cause of reproductive failure in birds, but predator identity often remains unknown. Ad- Received 12 May 2020 ditionally, although corvids are considered major nest predators in farmland landscapes, whether breeders or Received in revised form 9 July 2020 floaters are involved remains contentious. In this study, we aimed to identify nest predators using artificial nests, Accepted 9 July 2020 and test whether territorial or non-breeders carrion crow (Corvus corone) and Eurasian magpie (Pica pica) were Available online 17 July 2020 most likely involved. -

Printable PDF Format
Field Guides Tour Report Taiwan 2020 Feb 1, 2020 to Feb 12, 2020 Phil Gregory & local guide Arco Huang For our tour description, itinerary, past triplists, dates, fees, and more, please VISIT OUR TOUR PAGE. This gorgeous male Swinhoe's Pheasant was one of the birds of the trip! We found a pair of these lovely endemic pheasants at Dasyueshan. Photo by guide Phil Gregory. This was a first run for the newly reactivated Taiwan tour (which we last ran in 2006), with a new local organizer who proved very good and enthusiastic, and knew the best local sites to visit. The weather was remarkably kind to us and we had no significant daytime rain, somewhat to my surprise, whilst temperatures were pretty reasonable even in the mountains- though it was cold at night at Dasyueshan where the unheated hotel was a bit of a shock, but in a great birding spot, so overall it was bearable. Fog on the heights of Hohuanshan was a shame but at least the mid and lower levels stayed clear. Otherwise the lowland sites were all good despite it being very windy at Hengchun in the far south. Arco and I decided to use a varied assortment of local eating places with primarily local menus, and much to my amazement I found myself enjoying noodle dishes. The food was a highlight in fact, as it was varied, often delicious and best of all served quickly whilst being both hot and fresh. A nice adjunct to the trip, and avoided losing lots of time with elaborate meals. -

Hungary & Transylvania
Although we had many exciting birds, the ‘Bird of the trip’ was Wallcreeper in 2015. (János Oláh) HUNGARY & TRANSYLVANIA 14 – 23 MAY 2015 LEADER: JÁNOS OLÁH Central and Eastern Europe has a great variety of bird species including lots of special ones but at the same time also offers a fantastic variety of different habitats and scenery as well as the long and exciting history of the area. Birdquest has operated tours to Hungary since 1991, being one of the few pioneers to enter the eastern block. The tour itinerary has been changed a few times but nowadays the combination of Hungary and Transylvania seems to be a settled and well established one and offers an amazing list of European birds. This tour is a very good introduction to birders visiting Europe for the first time but also offers some difficult-to-see birds for those who birded the continent before. We had several tour highlights on this recent tour but certainly the displaying Great Bustards, a majestic pair of Eastern Imperial Eagle, the mighty Saker, the handsome Red-footed Falcon, a hunting Peregrine, the shy Capercaillie, the elusive Little Crake and Corncrake, the enigmatic Ural Owl, the declining White-backed Woodpecker, the skulking River and Barred Warblers, a rare Sombre Tit, which was a write-in, the fluty Red-breasted and Collared Flycatchers and the stunning Wallcreeper will be long remembered. We recorded a total of 214 species on this short tour, which is a respectable tally for Europe. Amongst these we had 18 species of raptors, 6 species of owls, 9 species of woodpeckers and 15 species of warblers seen! Our mammal highlight was undoubtedly the superb views of Carpathian Brown Bears of which we saw ten on a single afternoon! 1 BirdQuest Tour Report: Hungary & Transylvania 2015 www.birdquest-tours.com We also had a nice overview of the different habitats of a Carpathian transect from the Great Hungarian Plain through the deciduous woodlands of the Carpathian foothills to the higher conifer-covered mountains. -

WHITE-BELLIED HERON REPORT Ardea Insignis Hume, 1878
REPORT WHITE-BELLIED HERON Ardea insignis Hume, 1878 ANNUAL POPULATION SURVEY 2021 Report prepared by Indra Acharja/RSPN [email protected] Summary Introduction The 19 th White-bellied Heron (WBH) annual population survey conducted from 27 February – 03 March 2021 The White-bellied Heron (Ardea insignis Hume 1878) is a large heron species of the family Ardeidae, order counted 22 herons in the country. The survey confirmed 19 adults and three sub-adult individuals, which is five less Pelecaniformes, found in freshwater ecosystems of the Himalayas. It is categorized as critically endangered under than the previous year. The decrease in population was mainly observed in upper Punatsangchhu basin; Phochu, the IUCN Red List of threatened species and protected under the Schedule I of Forests and Nature Conservation Mochhu, Adha and Harachhu which were oldest and previously the most abundantly used habitats in Bhutan. The Act 1995 of Bhutan. It was listed as threatened in 1988, uplisted to endangered in 1994, and to critically endangered survey covered all currently known and expected habitats along Punatsangchhu, Mangdechhu, Chamkharchhu, since 2007. The distribution of WBH to undisturbed freshwater river systems and its piscivorous feeding behaviour Drangmechhu, Kurichhu, Kholongchhu and major tributaries. For the survey, habitats across the country were can be easily associated with the health of the ecosystem and pristinely environment. They are the indicators of our divided into 53 priority zones and surveyors were deployed to look for the WBH from 7:00 AM to 5:00 PM for freshwater river systems. Their presence in our rivers indicates the health of the rivers, the fish population, water five consecutive days within their designated zone. -

The Effect of Intense Light on Bird Behavior and Physiology
View metadata, citation and similar papers at core.ac.uk brought to you by CORE provided by DigitalCommons@University of Nebraska University of Nebraska - Lincoln DigitalCommons@University of Nebraska - Lincoln Wildlife Damage Management, Internet Center Bird Control Seminars Proceedings for October 1973 THE EFFECT OF INTENSE LIGHT ON BIRD BEHAVIOR AND PHYSIOLOGY Sheldon Lustick Ohio State University Follow this and additional works at: https://digitalcommons.unl.edu/icwdmbirdcontrol Part of the Environmental Sciences Commons Lustick, Sheldon, "THE EFFECT OF INTENSE LIGHT ON BIRD BEHAVIOR AND PHYSIOLOGY" (1973). Bird Control Seminars Proceedings. 119. https://digitalcommons.unl.edu/icwdmbirdcontrol/119 This Article is brought to you for free and open access by the Wildlife Damage Management, Internet Center for at DigitalCommons@University of Nebraska - Lincoln. It has been accepted for inclusion in Bird Control Seminars Proceedings by an authorized administrator of DigitalCommons@University of Nebraska - Lincoln. 171 THE EFFECT OF INTENSE LIGHT ON BIRD BEHAVIOR AND PHYSIOLOGY Sheldon Lustick Zoology Department Ohio State University , Columbus , Ohio 43210 It has been known for centuries that light (photoperiod ) is possibly the major environmental stimuli affecting bird behavior and physiology. The length of the light period stimulates the breeding cycle , migration , fat de - position , and molt in most species of birds. Therefore , it is only natural that one would think of using light as a means of bird control. In fa ct , light has already been used as a bird control; flood -light traps have been used to trap blackbirds (Meanley 1971 ); Meanley states that 2000 -W search lights have been used to alleviate depredation by ducks in rice fields. -

Bird Damage to Pistachios
The extent of damage to pistachios by some birds that knock nuts to the ground, where they hull, shell, and eat them, can be measured. Losses to birds that pluck nuts from the tree and fly off to eat them else- where can only be estimated. counties to the south. District I1 (Central) is Merced, Madera, Fresno, and Kings Bird damage to nistachios counties. District I11 (Northern) is Monte- rey, San Benito, Inyo, and all counties to the north of Merced County. Terrell P. Salmon 0 A. Charles Crabb 0 RexE.Marsh Scope of the problem We received 105 responses (23 percent) from the 458 surveys mailed. Thirteen (12.7 percent) were excluded from analy- Crows are the primary culprits sis, because the orchards represented followed by ravens and jays were not in production, were outside Cali- fornia, or were managed by another per- son. The remaining 92 indicated they had pistachio losses due to one or more bird species. Bird damage was widespread through- out the state, as indicated by surveys re- turned from 18 counties. These 18 coun- ties represent 98 percent of the bearing pistachio acreage in California. The infor- mation we report here is based on the sur- vey returns and does not account for bird Various bird species are pests to a step in defining the problem and evaluat- damage and control that undoubtedly oc- number of California crops. Nut crops ing current bird control methods. cur but were not reported. Our estimates such as pistachios, almonds, and walnuts The major focus of the survey was to should therefore be considered conserva- are particularly hard hit, although infor- identify the bird species involved, the ex- tive. -

Records of Four Critically Endangered Songbirds in the Markets of Java Suggest Domestic Trade Is a Major Impediment to Their Conservation
20 BirdingASIA 27 (2017): 20–25 CONSERVATION ALERT Records of four Critically Endangered songbirds in the markets of Java suggest domestic trade is a major impediment to their conservation VINCENT NIJMAN, SUCI LISTINA SARI, PENTHAI SIRIWAT, MARIE SIGAUD & K. ANNEISOLA NEKARIS Introduction 1.2 million wild-caught birds (the vast majority Bird-keeping is a popular pastime in Indonesia, and of them songbirds) were sold in the Java and Bali nowhere more so than amongst the people of Java. markets each year. Taking a different approach, It has deep cultural roots, and traditionally a kukilo Jepson & Ladle (2005) made use of a survey of (bird in the Javanese language) was one of the five randomly selected households in the Javan cities things a Javanese man should pursue or obtain in of Jakarta, Bandung, Semarang and Surabaya, order to live a fulfilling life (the others being garwo, and Medan in Sumatra, which together make up a wife, curigo, a Javanese dagger, wismo, a house or a quarter of the urban Indonesian population, to a place to live, and turonggo, a horse, as a means estimate that between 600,000 and 760,000 wild- of transportation). A kukilo represents having a caught native songbirds were acquired each year. hobby, and it often takes the form of owning a Extrapolating this to the urban population of Java, perkutut (Zebra Dove Geopelia striata) or a kutilang which amounts to 60% of Indonesia’s total, it (Sooty-headed Bulbul Pycnonotus aurigaster) but suggests that a total of 1.4–1.8 million wild-caught also a wide range of other birds (Nash 1993, Chng native songbirds were acquired. -

Pica (Pica) Bottanensis in India
PRŷS-JONES & RASMUSSEN: Black-rumped Magpie 71 The status of the Black-rumped Magpie Pica (pica) bottanensis in India Robert P. Prŷs-Jones & Pamela C. Rasmussen Prŷs-Jones, R. P., & Rasmussen, P. C., 2018. The status of the Black-rumped Magpie Pica (pica) bottanensis in India. Indian BIRDS 14 (3): 71–73. Robert P. Prŷs-Jones, Bird Group, Department of Life Sciences, Natural History Museum, Akeman St, Tring, Herts HP23 6AP, UK. E-mail: [email protected] [RPP-J] Pamela C. Rasmussen, Department of Integrative Biology and MSU Museum, Michigan State University, East Lansing, MI 48864, USA; Bird Group, Department of Life Sciences, Natural History Museum, Akeman St, Tring, Herts HP23 6AP, UK. E-mail: [email protected] [PCR] Manuscript received on 01 February 2018. he presence of the Eurasian Magpie Pica pica (sensu lato) in India (Praveen within Native Sikkim, any such records having et al. 2016) is predominantly based on the well-documented occurrence of more probably been a mistake for southern Tthe race bactriana in the north-western Himalayas east to northern Himachal Tibet,” (Meinertzhagen 1927: 371). There is Pradesh (Rasmussen & Anderton 2012; Dickinson & Christidis 2014). However, thus a clear contradiction between his own the question as to whether the taxon bottanensis may additionally occur, or have writings and the existence of his specimen, occurred, in Sikkim has recently resurfaced as a result of a comprehensive molecular from which we deduce that he most likely phylogenetic study of the genus Pica by Song et al. (2018), who recognised Pica (p.) stole the specimen later, relabelling it without bottanensis to be an anciently diverged and distinctive lineage. -

Magpie Jay General Information and Care
Magpie Jay General Information and Care: Black Throated Magpie Jays (Calocitta colliei) and White Throated Magpie Jay (Calocitta formosa) are the only two species in their genus. Black Throated Magpie Jays are endemic to Northwestern Mexico. The range of White Throated Magpie Jays lies to the south, overlapping with Black Throats slightly in the Mexican states of Jalisco and Colima, and running into Costa Rica. Both of these birds are members of the family Corvidae. Magpie Jays are energetic, highly intelligent animals and need to be kept in a large planted aviary - not just a cage. These birds are highly social and are commonly found in the wild as cooperative nesters. They are omnivores and favor a great variety of fruits, insects, small rodents, and nuts. A captive diet that works well in my aviaries is a basic pellet low iron softbill diet such as Kaytee’s Exact Original Low Iron Maintenance Formula for Toucans, Mynas and other Softbills. A bowl of this is in the cage at all times and is supplemented with nuts, fruits and veggies like apples, papaya, grapes, oranges, peas and carrots and the occasional treat of small mice and insects like meal worms, crickets, megaworms, and waxworms are relished by the birds. Extra protein is essential if you want these birds to breed. Fresh water should always be provided. I use a shallow three to four inch deep, twelve inch wide crock the birds can drink from and bath in. The birds you are receiving from my aviaries are hand fed and closed banded. How recently they were weaned will affect how tame they are at first. -
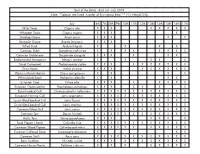
Best of the Baltic - Bird List - July 2019 Note: *Species Are Listed in Order of First Seeing Them ** H = Heard Only
Best of the Baltic - Bird List - July 2019 Note: *Species are listed in order of first seeing them ** H = Heard Only July 6th 7th 8th 9th 10th 11th 12th 13th 14th 15th 16th 17th Mute Swan Cygnus olor X X X X X X X X Whopper Swan Cygnus cygnus X X X X Greylag Goose Anser anser X X X X X Barnacle Goose Branta leucopsis X X X Tufted Duck Aythya fuligula X X X X Common Eider Somateria mollissima X X X X X X X X Common Goldeneye Bucephala clangula X X X X X X Red-breasted Merganser Mergus serrator X X X X X Great Cormorant Phalacrocorax carbo X X X X X X X X X X Grey Heron Ardea cinerea X X X X X X X X X Western Marsh Harrier Circus aeruginosus X X X X White-tailed Eagle Haliaeetus albicilla X X X X Eurasian Coot Fulica atra X X X X X X X X Eurasian Oystercatcher Haematopus ostralegus X X X X X X X Black-headed Gull Chroicocephalus ridibundus X X X X X X X X X X X X European Herring Gull Larus argentatus X X X X X X X X X X X X Lesser Black-backed Gull Larus fuscus X X X X X X X X X X X X Great Black-backed Gull Larus marinus X X X X X X X X X X X X Common/Mew Gull Larus canus X X X X X X X X X X X X Common Tern Sterna hirundo X X X X X X X X X X X X Arctic Tern Sterna paradisaea X X X X X X X Feral Pigeon ( Rock) Columba livia X X X X X X X X X X X X Common Wood Pigeon Columba palumbus X X X X X X X X X X X Eurasian Collared Dove Streptopelia decaocto X X X Common Swift Apus apus X X X X X X X X X X X X Barn Swallow Hirundo rustica X X X X X X X X X X X Common House Martin Delichon urbicum X X X X X X X X White Wagtail Motacilla alba X X -

The Molecular Evolution of Feathers with Direct Evidence from Fossils
The molecular evolution of feathers with direct evidence from fossils Yanhong Pana,1, Wenxia Zhengb, Roger H. Sawyerc, Michael W. Penningtond, Xiaoting Zhenge,f, Xiaoli Wange,f, Min Wangg,h, Liang Hua,i, Jingmai O’Connorg,h, Tao Zhaoa, Zhiheng Lig,h, Elena R. Schroeterb, Feixiang Wug,h, Xing Xug,h, Zhonghe Zhoug,h,i,1, and Mary H. Schweitzerb,j,1 aChinese Academy of Sciences Key Laboratory of Economic Stratigraphy and Palaeogeography, Nanjing Institute of Geology and Palaeontology and Center for Excellence in Life and Paleoenvironment, Chinese Academy of Sciences, Nanjing 210008, China; bDepartment of Biological Sciences, North Carolina State University, Raleigh, NC 27695; cDepartment of Biological Sciences, University of South Carolina, Columbia, SC 29205; dAmbioPharm Incorporated, North Augusta, SC 29842; eInstitute of Geology and Paleontology, Lingyi University, Lingyi City, 27605 Shandong, China; fShandong Tianyu Museum of Nature, Pingyi, 273300 Shandong, China; gCAS Key Laboratory of Vertebrate Evolution and Human Origins of the Chinese Academy of Sciences, Institute of Vertebrate Paleontology and Paleoanthropology, Chinese Academy of Sciences, 100044 Beijing, China; hCenter for Excellence in Life and Paleoenvironment, Chinese Academy of Sciences, 100044 Beijing, China; iCollege of Earth and Planetary Sciences, University of Chinese Academy of Sciences, 100049 Beijing, China; and jNorth Carolina Museum of Natural Sciences, Raleigh, NC 27601 Contributed by Zhonghe Zhou, December 15, 2018 (sent for review September 12, 2018; reviewed by Dominique G. Homberger and Chenxi Jia) Dinosaur fossils possessing integumentary appendages of various feathers in Anchiornis, barbules that interlock to form feather morphologies, interpreted as feathers, have greatly enhanced our vanes critical for flight have not been identified yet (12). -
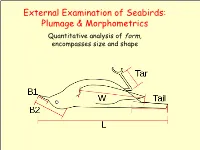
External Examination of Seabirds: Plumage & Morphometrics
External Examination of Seabirds: Plumage & Morphometrics Quantitative analysis of form, encompasses size and shape Seabird Topography ➢ Naming Conventions: • Parts of the body • Types of feathers (Harrison 1983) Types of Feathers Coverts: Rows bordering and overlaying the edges of the tail and wings on both the lower and upper sides of the body. Help streamline shape of the wings and tail and provide the bird with insulation. Feather Tracks ➢ Feathers are not attached randomly. • They occur in linear tracts called pterylae. • Spaces on bird's body without feather tracts are called apteria. • Densest area for feather tracks is head and neck. • Feathers arranged in distinct layers: contour feathers overlay down. Generic Pterylae Types of Feathers Contour feathers: outermost feathers. Define the color and shape of the bird. Contour feathers lie on top of each other, like shingles on a roof. Shed water, keeping body dry and insulated. Each contour feather controlled by specialized muscles which control their position, allowing the bird to keep the feathers in clean and neat condition. Specialized contour feathers used for flight: delineate outline of wings and tail. Types of Feathers Flight feathers – special contour feathers Define outline of wings and tail Long and stiff Asymmetrical those on wings are called remiges (singular remex) those on tail are called retrices (singular retrix) Types of Flight Feathers Remiges: Largest contour feathers (primaries / secondaries) Responsible for supporting bird during flight. Attached by ligaments or directly to the wing bone. Types of Flight Feathers Flight feathers – special contour feathers Rectrices: tail feathers provide flight stability and control. Connected to each other by ligaments, with only the inner- most feathers attached to bone.