54 Subpart F—Flavoring Agents and Related
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Retention Indices for Frequently Reported Compounds of Plant Essential Oils
Retention Indices for Frequently Reported Compounds of Plant Essential Oils V. I. Babushok,a) P. J. Linstrom, and I. G. Zenkevichb) National Institute of Standards and Technology, Gaithersburg, Maryland 20899, USA (Received 1 August 2011; accepted 27 September 2011; published online 29 November 2011) Gas chromatographic retention indices were evaluated for 505 frequently reported plant essential oil components using a large retention index database. Retention data are presented for three types of commonly used stationary phases: dimethyl silicone (nonpolar), dimethyl sili- cone with 5% phenyl groups (slightly polar), and polyethylene glycol (polar) stationary phases. The evaluations are based on the treatment of multiple measurements with the number of data records ranging from about 5 to 800 per compound. Data analysis was limited to temperature programmed conditions. The data reported include the average and median values of retention index with standard deviations and confidence intervals. VC 2011 by the U.S. Secretary of Commerce on behalf of the United States. All rights reserved. [doi:10.1063/1.3653552] Key words: essential oils; gas chromatography; Kova´ts indices; linear indices; retention indices; identification; flavor; olfaction. CONTENTS 1. Introduction The practical applications of plant essential oils are very 1. Introduction................................ 1 diverse. They are used for the production of food, drugs, per- fumes, aromatherapy, and many other applications.1–4 The 2. Retention Indices ........................... 2 need for identification of essential oil components ranges 3. Retention Data Presentation and Discussion . 2 from product quality control to basic research. The identifi- 4. Summary.................................. 45 cation of unknown compounds remains a complex problem, in spite of great progress made in analytical techniques over 5. -

UPDATED 18Th February 2013
7th February 2015 Welcome to my new seed trade list for 2014-15. 12, 13 and 14 in brackets indicates the harvesting year for the seed. Concerning seed quantity: as I don't have many plants of each species, seed quantity is limited in most cases. Therefore, for some species you may only get a few seeds. Many species are harvested in my garden. Others are surplus from trade and purchase. OUT: Means out of stock. Sometimes I sell surplus seed (if time allows), although this is unlikely this season. NB! Cultivars do not always come true. I offer them anyway, but no guarantees to what you will get! Botanical Name (year of harvest) NB! Traditional vegetables are at the end of the list with (mostly) common English names first. Acanthopanax henryi (14) Achillea sibirica (13) Aconitum lamarckii (12) Achyranthes aspera (14, 13) Adenophora khasiana (13) Adenophora triphylla (13) Agastache anisata (14,13)N Agastache anisata alba (13)N Agastache rugosa (Ex-Japan) (13) (two varieties) Agrostemma githago (13)1 Alcea rosea “Nigra” (13) Allium albidum (13) Allium altissimum (Persian Shallot) (14) Allium atroviolaceum (13) Allium beesianum (14,12) Allium brevistylum (14) Allium caeruleum (14)E Allium carinatum ssp. pulchellum (14) Allium carinatum ssp. pulchellum album (14)E Allium carolinianum (13)N Allium cernuum mix (14) E/N Allium cernuum “Dark Scape” (14)E Allium cernuum ‘Dwarf White” (14)E Allium cernuum ‘Pink Giant’ (14)N Allium cernuum x stellatum (14)E (received as cernuum , but it looks like a hybrid with stellatum, from SSE, OR KA A) Allium cernuum x stellatum (14)E (received as cernuum from a local garden centre) Allium clathratum (13) Allium crenulatum (13) Wild coll. -

Visible-Light-Driven Photooxidation of Alcohols Using Surface-Doped Graphitic Carbon Nitride
Electronic Supplementary Material (ESI) for Green Chemistry. This journal is © The Royal Society of Chemistry 2017 Visible-Light-Driven Photooxidation of alcohols using surface-doped graphitic carbon nitride Wuyuan Zhang, Anna Bariotaki, Ioulia Smonou, and Frank Hollmann* Material and Methods Materials Unless stated otherwise all chemicals were purchased from Sigma-Aldrich, Fluka, Acros or Alfa-Aesar with the highest purity available and used without further treatment. Synthesis of g-C3N4 and carbon nanodots doped (CD-C3N4) The g-C3N4 was synthesized via the simple calcination (Carbolite furnace; CWF 12/5, 2400 W) of urea (10.0 g) at 600 °C for 4 h (5 °C/min), a yellowish powder was obtained after the cooling to room temperature.1 The carbon nanodots were synthesized via the thermal decomposition of sucrose with slight modification.2 Briefly, 0.75 g of sucrose was dissolved in 30 mL of MilliQ water and stirred at room temperature for 0.5 h. The solution was transferred into a 45 mL Teflon-lined stainless steel autoclave reactor, and heated at 180 °C for 5 h (10 °C/min). After cooling the reactor to room temperature, a brown mixture was obtained. The mixture was centrifuged (8000 rpm for 20 min), and the pellet was washed with water (3×) and freeze-dried. 3 In order to deposit carbon nanodots to the surface of g-C3N4, Liu et al’s procedures were adopted. Firstly, a stock solution of carbon nanodots (1 mg in 25mL water) was prepared. 15.0 mL of this stock solution was mixed with 15.0 mL of NH4OH (28 %) and sealed in a 45 mL Teflon-lined autoclave reactor. -

(12) United States Patent (10) Patent No.: US 7,576,170 B2 Perry Et Al
US00757617OB2 (12) United States Patent (10) Patent No.: US 7,576,170 B2 Perry et al. (45) Date of Patent: Aug. 18, 2009 (54) CYCLIC SILOXANE COMPOSITIONS FOR 6,046,156 A 4/2000 Perry THE RELEASE OF ACTIVE INGREDIENTS 6,054,547 A 4/2000 Perry et al. 6,063,365 A 5, 2000 Schefer et al. (75) Inventors: Robert J. Perry, Niskayuna, NY (US); 6,075,111 A 6/2000 Perry et al. Mark D. Leatherman, Elmsford, NY 6,077.923 A 6/2000 Perry et al. (US); Shahid Murtuza, Albany, NY 6,083,901 A 7/2000 Perry et al. (US) 6,121,343 A 9/2000 Hongo et al. 6,143,309 A 11/2000 Legrow et al. (73) Assignee: Momentive Performance Materials, 6,153,578 A 11/2000 Perry Albany, NY (US) 6,200,949 B1 3/2001 Reijmer et al. 6,228,380 B1 5, 2001 LeGrow et al. (*) Notice: Subject to any disclaimer, the term of this 6,262,287 B1 7/2001 Anderson et al. patent is extended or adjusted under 35 6,267,977 B1 7/2001 LeGrow et al. U.S.C. 154(b) by 993 days. 6,309,715 B1 10/2001 Lindauer et al. 6,322,777 B1 1 1/2001 Perry et al. (21) Appl. No.: 10/742,415 6,325,274 B2 12/2001 Esumi et al. 6,325,859 B1 12/2001 De Roos et al. (22) Filed: Dec. 19, 2003 6,435,423 B2 8/2002 Hurry et al. (65) Prior Publication Data 6,624,136 B2 9, 2003 Guerinet al. -

Akshi Bansal* B. K. Dangarh ABSTRACT KEYWORDS
ORIGINAL RESEARCH PAPER Volume-7 | Issue-1 | January-2018 | PRINT ISSN No 2277 - 8179 INTERNATIONAL JOURNAL OF SCIENTIFIC RESEARCH OXIDATION STUDY OF 4-METHOXYBENZYL ALCOHOL BY CR (VI) OXIDANTS IN PARTIAL AQUEOUS MEDIUM- A COMPARATIVE STUDY Chemistry Research Scholar, Pacific academy of Higher Education and Research University, Akshi Bansal* Udaipur *Corresponding Author B. K. Dangarh Asstt. Prof. of Chemistry, Govt. P. G. College, Neemuch, M.P. ABSTRACT Oxidation of 4-Methoxybenzyl alcohol by different Cr (VI) oxidants [PDC and PCC] has been studied in acetic acid-H2O medium in the presence of PTSA spectrophotometrically. The reactions are found first order with respect to both the oxidants, [H+], and [substrate].Michaelis-Menten type kinetics is observed. The reaction rate decreases with increasing volume percentage of acetic acid in reaction mixture. The reaction was studied at different temperature [298-318 K] and activation parameters were computed. The oxidation product was identified as Cr (III) and corresponding aldehyde. The oxidation rate order was found with respect to the oxidants are: PCC > PDC KEYWORDS Oxidation, 4-Methoxybenzyl alcohol, PTSA, PCC and PDC. 1. INTRODUCTION The kinetics run were followed for more than 60-70% completion of 4-Methoxybenzyl alcohol (Anisyl alcohol) is used as a fragrance and the reaction and good first order kinetics were observed. flavouring. It occurs naturally but is produced by reduction of anisaldehyde. 3. RESULTS AND DISCUSSION: 3.1 Stoichiometry and product analysis: Kinetics and mechanism of the oxidation of substituted benzyl To determine the stoichiometry of a reaction a known slight excess of alcohols by pyridinium chlorochromate studied by Banerji[1] et al. -

Seedling Establishment, Bud Movement, and Subterranean Diversity of Geophilous Systems in Apiaceae
Flora (2002) 197, 385–393 http://www.urbanfischer.de/journals/flora Seedling establishment, bud movement, and subterranean diversity of geophilous systems in Apiaceae Norbert Pütz1* & Ina Sukkau2 1 Institute of Nature Conservation and Environmental Education, University of Vechta, Driverstr. 22, D-49377 Vechta, Germany 2 Institute of Botany, RWTH Aachen, Germany * author for correspondence: e-mail: [email protected] Received: Nov 29, 2001 · Accepted: Jun 10, 2002 Summary Geophilous systems of plants are not only regarded as organs of underground storage. Such systems also undergo a large range of modifications in order to fulfill other ‚cryptical‘ functions, e.g. positioning of innovation buds, vegetative cloning, and vege- tative dispersal. Seedlings should always be the point of departure for any investigation into the structure of geophilous systems. This is because in the ability to survive of geophilous plants it is of primary importance that innovation buds can reach a safe position in the soil by the time the first period hostile to vegetation commences. Our analysis of such systems thus focused on examining the development of 34 species of the Apiaceae, beginning with their germination. Independent of life-form and life-span, all species exhibit noticeable terminal bud movement with the aid of contractile organs. Movement was found to be at least 5 mm, reaching a maximum of 45 mm. All species exhibit a noticeable contraction of the primary root. In most cases the contraction phenomenon also occurs in the hypocotyl, and some species show contraction of their lateral and / or adventitious roots. Analysis of movement shows the functional importance of pulling the inno- vation buds down into the soil. -
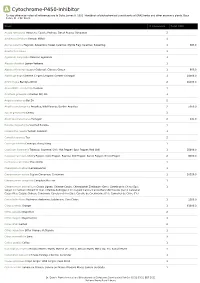
Show Activity
A Cytochrome-P450-Inhibitor *Unless otherwise noted all references are to Duke, James A. 1992. Handbook of phytochemical constituents of GRAS herbs and other economic plants. Boca Raton, FL. CRC Press. Plant # Chemicals Total PPM Acacia farnesiana Huisache; Cassie; Popinac; Sweet Acacia; Opopanax 2 Achillea millefolium Yarrow; Milfoil 1 Acorus calamus Flagroot; Sweetroot; Sweet Calamus; Myrtle Flag; Calamus; Sweetflag 1 384.0 Agastache rugosa 1 Ageratum conyzoides Mexican ageratum 1 Aloysia citrodora Lemon Verbena 1 Alpinia officinarum Lesser Galangal; Chinese Ginger 1 800.0 Alpinia galanga Siamese Ginger; Languas; Greater Galangal 1 24000.0 Ammi majus Bishop's Weed 2 16000.0 Anacardium occidentale Cashew 1 Anethum graveolens Garden Dill; Dill 1 Angelica dahurica Bai Zhi 2 Angelica archangelica Angelica; Wild Parsnip; Garden Angelica 2 5050.0 Apium graveolens Celery 3 Artemisia dracunculus Tarragon 2 141.0 Boronia megastigma Scented Boronia 1 Calamintha nepeta Turkish Calamint 1 Camellia sinensis Tea 2 Cananga odorata Cananga; Ylang-Ylang 1 Capsicum frutescens Tabasco; Cayenne; Chili; Hot Pepper; Spur Pepper; Red Chili 1 35800.0 Capsicum annuum Cherry Pepper; Cone Pepper; Paprika; Bell Pepper; Sweet Pepper; Green Pepper 2 8000.0 Centaurea calcitrapa Star-Thistle 1 Chenopodium album Lambsquarter 1 Cinnamomum verum Ceylon Cinnamon; Cinnamon 1 20320.0 Cinnamomum camphora Camphor; Ho Leaf 1 Cinnamomum aromaticum Cassia Lignea; Chinese Cassia; Chinesischer Zimtbaum (Ger.); Canela de la China (Sp.); 1 Saigon Cinnamon; Chinazimt (Ger.); Kashia-Keihi -

Peucedanum Ostruthium Inhibits E-Selectin and VCAM-1 Expression in Endothelial Cells Through Interference with NF-Κb Signaling
biomolecules Article Peucedanum ostruthium Inhibits E-Selectin and VCAM-1 Expression in Endothelial Cells through Interference with NF-κB Signaling Christoph Lammel 1, Julia Zwirchmayr 2 , Jaqueline Seigner 1 , Judith M. Rollinger 2,* and Rainer de Martin 1 1 Department of Vascular Biology and Thrombosis Research, Medical University of Vienna, Schwarzspanierstaße 17, 1090 Vienna, Austria; [email protected] (C.L.); [email protected] (J.S.); [email protected] (R.d.M.) 2 Department of Pharmacognosy, Faculty of Life Sciences, University of Vienna, Althanstraße 14, 1090 Vienna, Austria; [email protected] * Correspondence: [email protected]; Tel.: +43-1-4277-55255; Fax: +43-1-4277-855255 Received: 5 June 2020; Accepted: 18 August 2020; Published: 21 August 2020 Abstract: Twenty natural remedies traditionally used against different inflammatory diseases were probed for their potential to suppress the expression of the inflammatory markers E-selectin and VCAM-1 in a model system of IL-1 stimulated human umbilical vein endothelial cells (HUVEC). One third of the tested extracts showed in vitro inhibitory effects comparable to the positive control oxozeaenol, an inhibitor of TAK1. Among them, the extract derived from the roots and rhizomes of Peucedanum ostruthium (i.e., Radix Imperatoriae), also known as masterwort, showed a pronounced and dose-dependent inhibitory effect. Reporter gene analysis demonstrated that inhibition takes place on the transcriptional level and involves the transcription factor NF-κB. A more detailed analysis revealed that the P. ostruthium extract (PO) affected the phosphorylation, degradation, and resynthesis of IκBα, the activation of IKKs, and the nuclear translocation of the NF-κB subunit RelA. -

European Patent Office of Opposition to That Patent, in Accordance with the Implementing Regulations
(19) TZZ __T (11) EP 2 416 766 B1 (12) EUROPEAN PATENT SPECIFICATION (45) Date of publication and mention (51) Int Cl.: of the grant of the patent: A61K 31/045 (2006.01) A61K 45/06 (2006.01) 09.04.2014 Bulletin 2014/15 A61K 8/34 (2006.01) A61P 17/00 (2006.01) A61P 17/16 (2006.01) A61Q 19/00 (2006.01) (2006.01) (2006.01) (21) Application number: 09701247.0 A23L 1/30 A23L 2/02 A23L 2/39 (2006.01) A23C 9/13 (2006.01) A23C 11/10 (2006.01) A61Q 5/02 (2006.01) (22) Date of filing: 09.04.2009 A61Q 11/00 (2006.01) A61Q 15/00 (2006.01) A61Q 17/04 (2006.01) A61Q 19/02 (2006.01) A61K 8/02 (2006.01) A61Q 19/04 (2006.01) (86) International application number: PCT/EP2009/054336 (87) International publication number: WO 2009/087242 (16.07.2009 Gazette 2009/29) (54) COMPOSITIONS COMPRISING TRANS-TERT-BUTYL CYCLOHEXANOL AS SKIN IRRITATION- REDUCING AGENT ZUSAMMENSETZUNGEN MIT TRANS-TERT-BUTYL-CYCLOHEXANOL ALS MITTEL ZUR REDUZIERUNG VON HAUTREIZUNGEN COMPOSITIONS COMPRENANT DU TRANS-TERT-BUTYL CYCLOHEXANOL EN TANT QU’AGENT RÉDUISANT L’IRRITATION CUTANÉE (84) Designated Contracting States: • KROHN, Michael AT BE BG CH CY CZ DE DK EE ES FI FR GB GR 64653 Lorsch (DE) HR HU IE IS IT LI LT LU LV MC MK MT NL NO PL • ZINKE, Holger PT RO SE SI SK TR 64646 Heppenheim (DE) (43) Date of publication of application: (74) Representative: Eisenführ Speiser 15.02.2012 Bulletin 2012/07 Patentanwälte Rechtsanwälte PartGmbB Postfach 10 60 78 (73) Proprietor: Symrise AG 28060 Bremen (DE) 37603 Holzminden (DE) (56) References cited: (72) Inventors: EP-A2- 0 755 910 WO-A1-97/22332 • VIELHABER, Gabriele WO-A1-2008/117254 US-A- 2 927 127 75008 Paris (FR) • OERTLING, Heiko • SYMRISE GMBH & CO KG ET AL: "Trans-tert- 1012 Lausanne (CH) butyl cyclohexanol as skin irritation-reducing • GÖMANN, Claudia agent" RESEARCH DISCLOSURE, MASON 37640 Warbsen (DE) PUBLICATIONS, HAMPSHIRE, GB, vol. -

Food and Drug Administration, HHS § 172.515
Food and Drug Administration, HHS § 172.515 Common name Scientific name Limitations Tolu ................................................................ Myroxylon balsamum (L.) Harms. Turpentine ...................................................... Pinus palustris Mill. and other Pinus spp. which yield terpene oils exclusively. Valerian rhizome and roots ............................ Valeriana officinalis L. Veronica ......................................................... Veronica officinalis L .................................................. Do. Vervain, European ......................................... Verbena officinalis L ................................................... Do. Vetiver ............................................................ Vetiveria zizanioides Stapf ......................................... Do. Violet, Swiss ................................................... Viola calcarata L. Walnut husks (hulls), leaves, and green nuts Juglans nigra L. or J. regia L. Woodruff, sweet ............................................. Asperula odorata L ..................................................... In alcoholic beverages only Yarrow ............................................................ Achillea millefolium L .................................................. In beverages only; fin- ished beverage thujone free1 Yerba santa .................................................... Eriodictyon californicum (Hook, et Arn.) Torr. Yucca, Joshua-tree ........................................ Yucca brevifolia Engelm. Yucca, -

Floristic Quality Assessment Report
FLORISTIC QUALITY ASSESSMENT IN INDIANA: THE CONCEPT, USE, AND DEVELOPMENT OF COEFFICIENTS OF CONSERVATISM Tulip poplar (Liriodendron tulipifera) the State tree of Indiana June 2004 Final Report for ARN A305-4-53 EPA Wetland Program Development Grant CD975586-01 Prepared by: Paul E. Rothrock, Ph.D. Taylor University Upland, IN 46989-1001 Introduction Since the early nineteenth century the Indiana landscape has undergone a massive transformation (Jackson 1997). In the pre-settlement period, Indiana was an almost unbroken blanket of forests, prairies, and wetlands. Much of the land was cleared, plowed, or drained for lumber, the raising of crops, and a range of urban and industrial activities. Indiana’s native biota is now restricted to relatively small and often isolated tracts across the State. This fragmentation and reduction of the State’s biological diversity has challenged Hoosiers to look carefully at how to monitor further changes within our remnant natural communities and how to effectively conserve and even restore many of these valuable places within our State. To meet this monitoring, conservation, and restoration challenge, one needs to develop a variety of appropriate analytical tools. Ideally these techniques should be simple to learn and apply, give consistent results between different observers, and be repeatable. Floristic Assessment, which includes metrics such as the Floristic Quality Index (FQI) and Mean C values, has gained wide acceptance among environmental scientists and decision-makers, land stewards, and restoration ecologists in Indiana’s neighboring states and regions: Illinois (Taft et al. 1997), Michigan (Herman et al. 1996), Missouri (Ladd 1996), and Wisconsin (Bernthal 2003) as well as northern Ohio (Andreas 1993) and southern Ontario (Oldham et al. -

Dr. Duke's Phytochemical and Ethnobotanical Databases List of Plants for Lyme Disease (Chronic)
Dr. Duke's Phytochemical and Ethnobotanical Databases List of Plants for Lyme Disease (Chronic) Plant Chemical Count Activity Count Garcinia xanthochymus 1 1 Nicotiana rustica 1 1 Acacia modesta 1 1 Galanthus nivalis 1 1 Dryopteris marginalis 2 1 Premna integrifolia 1 1 Senecio alpinus 1 1 Cephalotaxus harringtonii 1 1 Comptonia peregrina 1 1 Diospyros rotundifolia 1 1 Alnus crispa 1 1 Haplophyton cimicidum 1 1 Diospyros undulata 1 1 Roylea elegans 1 1 Bruguiera gymnorrhiza 1 1 Gmelina arborea 1 1 Orthosphenia mexicana 1 1 Lumnitzera racemosa 1 1 Melilotus alba 2 1 Duboisia leichhardtii 1 1 Erythroxylum zambesiacum 1 1 Salvia beckeri 1 1 Cephalotaxus spp 1 1 Taxus cuspidata 3 1 Suaeda maritima 1 1 Rhizophora mucronata 1 1 Streblus asper 1 1 Plant Chemical Count Activity Count Dianthus sp. 1 1 Glechoma hirsuta 1 1 Phyllanthus flexuosus 1 1 Euphorbia broteri 1 1 Hyssopus ferganensis 1 1 Lemaireocereus thurberi 1 1 Holacantha emoryi 1 1 Casearia arborea 1 1 Fagonia cretica 1 1 Cephalotaxus wilsoniana 1 1 Hydnocarpus anthelminticus 2 1 Taxus sp 2 1 Zataria multiflora 1 1 Acinos thymoides 1 1 Ambrosia artemisiifolia 1 1 Rhododendron schotense 1 1 Sweetia panamensis 1 1 Thymelaea hirsuta 1 1 Argyreia nervosa 1 1 Carapa guianensis 1 1 Parthenium hysterophorus 1 1 Rhododendron anthopogon 1 1 Strobilanthes cusia 1 1 Dianthus superbus 1 1 Pyropolyporus fomentarius 1 1 Euphorbia hermentiana 1 1 Porteresia coarctata 1 1 2 Plant Chemical Count Activity Count Aerva lanata 1 1 Rivea corymbosa 1 1 Solanum mammosum 1 1 Juniperus horizontalis 1 1 Maytenus