Peucedanum Ostruthium Inhibits E-Selectin and VCAM-1 Expression in Endothelial Cells Through Interference with NF-Κb Signaling
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

UPDATED 18Th February 2013
7th February 2015 Welcome to my new seed trade list for 2014-15. 12, 13 and 14 in brackets indicates the harvesting year for the seed. Concerning seed quantity: as I don't have many plants of each species, seed quantity is limited in most cases. Therefore, for some species you may only get a few seeds. Many species are harvested in my garden. Others are surplus from trade and purchase. OUT: Means out of stock. Sometimes I sell surplus seed (if time allows), although this is unlikely this season. NB! Cultivars do not always come true. I offer them anyway, but no guarantees to what you will get! Botanical Name (year of harvest) NB! Traditional vegetables are at the end of the list with (mostly) common English names first. Acanthopanax henryi (14) Achillea sibirica (13) Aconitum lamarckii (12) Achyranthes aspera (14, 13) Adenophora khasiana (13) Adenophora triphylla (13) Agastache anisata (14,13)N Agastache anisata alba (13)N Agastache rugosa (Ex-Japan) (13) (two varieties) Agrostemma githago (13)1 Alcea rosea “Nigra” (13) Allium albidum (13) Allium altissimum (Persian Shallot) (14) Allium atroviolaceum (13) Allium beesianum (14,12) Allium brevistylum (14) Allium caeruleum (14)E Allium carinatum ssp. pulchellum (14) Allium carinatum ssp. pulchellum album (14)E Allium carolinianum (13)N Allium cernuum mix (14) E/N Allium cernuum “Dark Scape” (14)E Allium cernuum ‘Dwarf White” (14)E Allium cernuum ‘Pink Giant’ (14)N Allium cernuum x stellatum (14)E (received as cernuum , but it looks like a hybrid with stellatum, from SSE, OR KA A) Allium cernuum x stellatum (14)E (received as cernuum from a local garden centre) Allium clathratum (13) Allium crenulatum (13) Wild coll. -

An Overview of Medicinal Plants As Potential Anti-Platelet Agents
IOSR Journal of Pharmacy and Biological Sciences (IOSR-JPBS) e-ISSN:2278-3008, p-ISSN:2319-7676. Volume 12, Issue 1 Ver. IV (Jan. - Feb.2017), PP 17-20 www.iosrjournals.org An Overview of Medicinal Plants as Potential Anti-Platelet Agents Mridul Bhowal1, Darshika M Mehta2 1B Pharm, PES College of Pharmacy, 50 feet road, Hanumanthanagar, Bengaluru-560050, Karnataka, India 2UG Scholar, PES College of Pharmacy, 50 feet road, Hanumanthanagar, Bengaluru-560050, Karnataka, India Abstract: Anti-platelet agents are those that decrease platelet aggregation and inhibit thrombus formation. Anti-platelet drugs are used to prevent and help in the reversal of platelet aggregation in arterial thrombosis which is the principal reason in the pathology of myocardial infarction (MI) and ischemic stroke. Currently many synthetic and semi-synthetic formulations are available in the markets which are potent anti-platelet agents but they have significant adverse effects. Herbs have been always an ideal source of drugs and numerous of the presently available drugs have been obtained directly or indirectly from them. In this article, authors made an extensive literature study about the work carried out on herbs with anti-platelet activity and their bioactive constituent. Here, an attempt was made to elaborate the isolated constituents from plant origin, which showed promising activity as anti-platelet agent. Keywords: Anti-platelet, Constituents, Extracts, Herbs, Medicinal Plants I. Introduction Platelets play an essential role in the initial response to vascular injury [1]. Its activation leads to formation of a haemostatic plug at the site of injury. Activation of platelets is therefore crucial for normal haemostasis but, uncontrolled platelet activation may also lead to the formation of occlusive thrombi that can cause ischemic events [2] and thus, anti-platelet therapy is very much needed for treatment. -

Flowering Plants Eudicots Apiales, Gentianales (Except Rubiaceae)
Edited by K. Kubitzki Volume XV Flowering Plants Eudicots Apiales, Gentianales (except Rubiaceae) Joachim W. Kadereit · Volker Bittrich (Eds.) THE FAMILIES AND GENERA OF VASCULAR PLANTS Edited by K. Kubitzki For further volumes see list at the end of the book and: http://www.springer.com/series/1306 The Families and Genera of Vascular Plants Edited by K. Kubitzki Flowering Plants Á Eudicots XV Apiales, Gentianales (except Rubiaceae) Volume Editors: Joachim W. Kadereit • Volker Bittrich With 85 Figures Editors Joachim W. Kadereit Volker Bittrich Johannes Gutenberg Campinas Universita¨t Mainz Brazil Mainz Germany Series Editor Prof. Dr. Klaus Kubitzki Universita¨t Hamburg Biozentrum Klein-Flottbek und Botanischer Garten 22609 Hamburg Germany The Families and Genera of Vascular Plants ISBN 978-3-319-93604-8 ISBN 978-3-319-93605-5 (eBook) https://doi.org/10.1007/978-3-319-93605-5 Library of Congress Control Number: 2018961008 # Springer International Publishing AG, part of Springer Nature 2018 This work is subject to copyright. All rights are reserved by the Publisher, whether the whole or part of the material is concerned, specifically the rights of translation, reprinting, reuse of illustrations, recitation, broadcasting, reproduction on microfilms or in any other physical way, and transmission or information storage and retrieval, electronic adaptation, computer software, or by similar or dissimilar methodology now known or hereafter developed. The use of general descriptive names, registered names, trademarks, service marks, etc. in this publication does not imply, even in the absence of a specific statement, that such names are exempt from the relevant protective laws and regulations and therefore free for general use. -

Seedling Establishment, Bud Movement, and Subterranean Diversity of Geophilous Systems in Apiaceae
Flora (2002) 197, 385–393 http://www.urbanfischer.de/journals/flora Seedling establishment, bud movement, and subterranean diversity of geophilous systems in Apiaceae Norbert Pütz1* & Ina Sukkau2 1 Institute of Nature Conservation and Environmental Education, University of Vechta, Driverstr. 22, D-49377 Vechta, Germany 2 Institute of Botany, RWTH Aachen, Germany * author for correspondence: e-mail: [email protected] Received: Nov 29, 2001 · Accepted: Jun 10, 2002 Summary Geophilous systems of plants are not only regarded as organs of underground storage. Such systems also undergo a large range of modifications in order to fulfill other ‚cryptical‘ functions, e.g. positioning of innovation buds, vegetative cloning, and vege- tative dispersal. Seedlings should always be the point of departure for any investigation into the structure of geophilous systems. This is because in the ability to survive of geophilous plants it is of primary importance that innovation buds can reach a safe position in the soil by the time the first period hostile to vegetation commences. Our analysis of such systems thus focused on examining the development of 34 species of the Apiaceae, beginning with their germination. Independent of life-form and life-span, all species exhibit noticeable terminal bud movement with the aid of contractile organs. Movement was found to be at least 5 mm, reaching a maximum of 45 mm. All species exhibit a noticeable contraction of the primary root. In most cases the contraction phenomenon also occurs in the hypocotyl, and some species show contraction of their lateral and / or adventitious roots. Analysis of movement shows the functional importance of pulling the inno- vation buds down into the soil. -
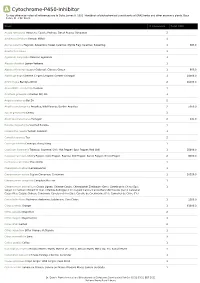
Show Activity
A Cytochrome-P450-Inhibitor *Unless otherwise noted all references are to Duke, James A. 1992. Handbook of phytochemical constituents of GRAS herbs and other economic plants. Boca Raton, FL. CRC Press. Plant # Chemicals Total PPM Acacia farnesiana Huisache; Cassie; Popinac; Sweet Acacia; Opopanax 2 Achillea millefolium Yarrow; Milfoil 1 Acorus calamus Flagroot; Sweetroot; Sweet Calamus; Myrtle Flag; Calamus; Sweetflag 1 384.0 Agastache rugosa 1 Ageratum conyzoides Mexican ageratum 1 Aloysia citrodora Lemon Verbena 1 Alpinia officinarum Lesser Galangal; Chinese Ginger 1 800.0 Alpinia galanga Siamese Ginger; Languas; Greater Galangal 1 24000.0 Ammi majus Bishop's Weed 2 16000.0 Anacardium occidentale Cashew 1 Anethum graveolens Garden Dill; Dill 1 Angelica dahurica Bai Zhi 2 Angelica archangelica Angelica; Wild Parsnip; Garden Angelica 2 5050.0 Apium graveolens Celery 3 Artemisia dracunculus Tarragon 2 141.0 Boronia megastigma Scented Boronia 1 Calamintha nepeta Turkish Calamint 1 Camellia sinensis Tea 2 Cananga odorata Cananga; Ylang-Ylang 1 Capsicum frutescens Tabasco; Cayenne; Chili; Hot Pepper; Spur Pepper; Red Chili 1 35800.0 Capsicum annuum Cherry Pepper; Cone Pepper; Paprika; Bell Pepper; Sweet Pepper; Green Pepper 2 8000.0 Centaurea calcitrapa Star-Thistle 1 Chenopodium album Lambsquarter 1 Cinnamomum verum Ceylon Cinnamon; Cinnamon 1 20320.0 Cinnamomum camphora Camphor; Ho Leaf 1 Cinnamomum aromaticum Cassia Lignea; Chinese Cassia; Chinesischer Zimtbaum (Ger.); Canela de la China (Sp.); 1 Saigon Cinnamon; Chinazimt (Ger.); Kashia-Keihi -

The Business Environment in Okinawa
The Business Environment in Okinawa The Bank of Okinawa,Ltd 3 People’s Bank Competitive Advantage of Okinawa’s Ideal Location With major Asian cities within range of 4 hours, located in the heart of East Asia Overnight freight network with Okinawa as the hub Freight network linking major cities Connections to ANA in Japan and Asia, centered on Various domestic and cities in international routes Naha Airport Asia Nagoya Kansai ~Jun. 4 Haneda North America, Europe Cargo freighter (B767-F) night Kitakyushu Narita services to major Asian cities Jun. 5 ~ Quick connections to major Various Various cities in Seoul Okinawa Taipei cities in Japanese cities via Haneda Asia Asia 21 direct domestic passenger Shanghai Singapore routes Various Inland China cities in Various cities in Guangzhou Bangkok Asia Asia Hong Offers express services within Asia Kong Asia Inland China Middle East Oceania Asia Middle East Oceania 2018 summer schedule Source: ANA Cargo The Bank of Okinawa,Ltd 4 People’s Bank Okinawa Growth Industry Strategy: Development as a Gateway to Asia Okinawa Growth Industry Strategy: Development as a Gateway to Asia State of Okinawa of State Points The Conference of Industrial Competitiveness in Kyushu and Okinawa was established An international air cargo hub business began in 2009 to connect various Asian cities to consider growth strategies in the Kyushu and Okinawa regions, in light of an with the mainland, utilizing the locational benefits of Okinawa. Okinawa is highly suited emergency resolution by the Japan Revitalization Strategy and National Governors’ as an industrial location targeting emerging markets. Association. A world-class research educational institution (OIST) was opened. -

Suppression of TPA-Induced Cancer Cell Invasion by Peucedanum Japonicum Thunb. Extract Through the Inhibition of Pkcα/NF-Κb-Dependent MMP-9 Expression in MCF-7 Cells
108 INTERNATIONAL JOURNAL OF MOLECULAR MEDICINE 37: 108-114, 2016 Suppression of TPA-induced cancer cell invasion by Peucedanum japonicum Thunb. extract through the inhibition of PKCα/NF-κB-dependent MMP-9 expression in MCF-7 cells JEONG-MI KIM1*, EUN-MI NOH1*, HA-RIM KIM1, MI-SEONG KIM1, HYUN-KYUNG SONG1, MINOK LEE1, SEI-HOON YANG2, GUEM-SAN LEE3, HYOUNG-CHUL MOON4, KANG-BEOM KWON1,5 and YOUNG-RAE LEE1,6 1Center for Metabolic Function Regulation, 2Department of Internal Medicine, Wonkwang University School of Medicine; 3Department of Herbology, Wonkwang University School of Korean Medicine, Iksan, Jeonbuk 570-749; 4Institute of Customized Physical Therapy, Gwanju Metropolitan City 506-303; 5Department of Korean Physiology, Wonkwang University School of Korean Medicine; 6Department of Oral Biochemistry and Institute of Biomaterial-Implant, School of Dentistry, Wonkwang University, Iksan, Jeonbuk 570-749, Republic of Korea Received April 14, 2015; Accepted November 16, 2015 DOI: 10.3892/ijmm.2015.2417 Abstract. Metastatic cancers spread from their site of origin Introduction (the primary site) to other parts of the body. Matrix metallopro- teinase-9 (MMP-9), which degrades the extracellular matrix, Breast cancer is one of the most common malignancies is important in metastatic cancers as it plays a major role in affecting women worldwide, and the second leading cause of cancer cell invasion. The present study examined the inhibi- cancer-related mortality in women (1). The majority of breast tory effect of an ethanol extract of Peucedanum japonicum cancer-related deaths are caused by distant metastases from Thunb. (PJT) on MMP-9 expression and the invasion of MCF-7 the primary tumor site. -

Philipp Simon Massimo Iorizzo Dariusz Grzebelus Rafal Baranski Editors the Carrot Genome Compendium of Plant Genomes
Compendium of Plant Genomes Philipp Simon Massimo Iorizzo Dariusz Grzebelus Rafal Baranski Editors The Carrot Genome Compendium of Plant Genomes Series Editor Chittaranjan Kole, ICAR-National Research Center on Plant Biotechnology, Pusa, Raja Ramanna Fellow, Government of India, New Delhi, India [email protected] Philipp Simon • Massimo Iorizzo • Dariusz Grzebelus • Rafal Baranski Editors The Carrot Genome 123 [email protected] Editors Philipp Simon Massimo Iorizzo Vegetable Crops Research Unit Plants for Human Health Institute USDA-ARS North Carolina State University Madison, WI, USA Kannapolis, NC, USA Dariusz Grzebelus Rafal Baranski University of Agriculture in Krakow Faculty of Biotechnology and Kraków, Poland Horticulture University of Agriculture in Krakow Kraków, Poland ISSN 2199-4781 ISSN 2199-479X (electronic) Compendium of Plant Genomes ISBN 978-3-030-03388-0 ISBN 978-3-030-03389-7 (eBook) https://doi.org/10.1007/978-3-030-03389-7 Library of Congress Control Number: 2019934354 © Springer Nature Switzerland AG 2019 This work is subject to copyright. All rights are reserved by the Publisher, whether the whole or part of the material is concerned, specifically the rights of translation, reprinting, reuse of illustrations, recitation, broadcasting, reproduction on microfilms or in any other physical way, and transmission or information storage and retrieval, electronic adaptation, computer software, or by similar or dissimilar methodology now known or hereafter developed. The use of general descriptive names, registered names, trademarks, service marks, etc. in this publication does not imply, even in the absence of a specific statement, that such names are exempt from the relevant protective laws and regulations and therefore free for general use. -

Essential Oil from Herb and Rhizome of Peucedanum Ostruthium (L. Koch.) Ex DC
Essential Oil from Herb and Rhizome of Peucedanum ostruthium (L. Koch.) ex DC. Wojciech Cisowskiad*, Urszula Sawicka3, Marek Mardarowiczb, Monika Asztemborskac and Maria Łuczkiewiczd a Department of Pharmacognosy, Wroclaw University of Medicine, pi. Nankiera 1, 50-140 Wroclaw, Poland. Fax: ++48713445830 b Department of Chemical Physics, Faculty of Chemistry, Maria Curie-Sklodowska University, sq. M. Curie-Sklodowskiej 3, 20-031 Lublin, Poland c Department of Chemical Physics, Polish Academy of Sciences, str. Kasprzaka 44\52, 01-224 Warszawa, Poland d Department of Pharmacognosy, Medical University of Gdansk, AI. Gen. J. Hallera 107, 80-416 Gdansk, Poland * Author for correspondence and reprint requests Z. Naturforsch. 56c, 930-932 (2001); received April 23/July 19, 2001 Peucedanum ostruthium, Essential Oil, Chiral Components Essential oil from herb and rhizome of Peucedanum ostruthium (L.Koch.) ex DC un derwent qualitative and quantitative analyses. The content of the oil obtained by hydrodistil lation was 0.95% in the herb and 1.25% in the rhizome (per dry weight basis). Gas chroma tography (GC) with MS detection and flame ionisation detection showed that the oil from the rhizome contains 39 compounds, of which 29 were identified. Gas chromatography with flame ionisation detection in chiral columns against standard compounds showed the pres ence of enantiomers of some of the components of the oil. Compounds present in largest quantities are: sabinene (35.2%) of which (+) sabinene accounts for (96.54%) and 4-terpineol (26.6%) of which (+) 4-terpineol accounts for (65.8%). 44 components were found in the herb essential oil, of which 39 compounds were identified. -

Floristic Quality Assessment Report
FLORISTIC QUALITY ASSESSMENT IN INDIANA: THE CONCEPT, USE, AND DEVELOPMENT OF COEFFICIENTS OF CONSERVATISM Tulip poplar (Liriodendron tulipifera) the State tree of Indiana June 2004 Final Report for ARN A305-4-53 EPA Wetland Program Development Grant CD975586-01 Prepared by: Paul E. Rothrock, Ph.D. Taylor University Upland, IN 46989-1001 Introduction Since the early nineteenth century the Indiana landscape has undergone a massive transformation (Jackson 1997). In the pre-settlement period, Indiana was an almost unbroken blanket of forests, prairies, and wetlands. Much of the land was cleared, plowed, or drained for lumber, the raising of crops, and a range of urban and industrial activities. Indiana’s native biota is now restricted to relatively small and often isolated tracts across the State. This fragmentation and reduction of the State’s biological diversity has challenged Hoosiers to look carefully at how to monitor further changes within our remnant natural communities and how to effectively conserve and even restore many of these valuable places within our State. To meet this monitoring, conservation, and restoration challenge, one needs to develop a variety of appropriate analytical tools. Ideally these techniques should be simple to learn and apply, give consistent results between different observers, and be repeatable. Floristic Assessment, which includes metrics such as the Floristic Quality Index (FQI) and Mean C values, has gained wide acceptance among environmental scientists and decision-makers, land stewards, and restoration ecologists in Indiana’s neighboring states and regions: Illinois (Taft et al. 1997), Michigan (Herman et al. 1996), Missouri (Ladd 1996), and Wisconsin (Bernthal 2003) as well as northern Ohio (Andreas 1993) and southern Ontario (Oldham et al. -

Dr. Duke's Phytochemical and Ethnobotanical Databases List of Plants for Lyme Disease (Chronic)
Dr. Duke's Phytochemical and Ethnobotanical Databases List of Plants for Lyme Disease (Chronic) Plant Chemical Count Activity Count Garcinia xanthochymus 1 1 Nicotiana rustica 1 1 Acacia modesta 1 1 Galanthus nivalis 1 1 Dryopteris marginalis 2 1 Premna integrifolia 1 1 Senecio alpinus 1 1 Cephalotaxus harringtonii 1 1 Comptonia peregrina 1 1 Diospyros rotundifolia 1 1 Alnus crispa 1 1 Haplophyton cimicidum 1 1 Diospyros undulata 1 1 Roylea elegans 1 1 Bruguiera gymnorrhiza 1 1 Gmelina arborea 1 1 Orthosphenia mexicana 1 1 Lumnitzera racemosa 1 1 Melilotus alba 2 1 Duboisia leichhardtii 1 1 Erythroxylum zambesiacum 1 1 Salvia beckeri 1 1 Cephalotaxus spp 1 1 Taxus cuspidata 3 1 Suaeda maritima 1 1 Rhizophora mucronata 1 1 Streblus asper 1 1 Plant Chemical Count Activity Count Dianthus sp. 1 1 Glechoma hirsuta 1 1 Phyllanthus flexuosus 1 1 Euphorbia broteri 1 1 Hyssopus ferganensis 1 1 Lemaireocereus thurberi 1 1 Holacantha emoryi 1 1 Casearia arborea 1 1 Fagonia cretica 1 1 Cephalotaxus wilsoniana 1 1 Hydnocarpus anthelminticus 2 1 Taxus sp 2 1 Zataria multiflora 1 1 Acinos thymoides 1 1 Ambrosia artemisiifolia 1 1 Rhododendron schotense 1 1 Sweetia panamensis 1 1 Thymelaea hirsuta 1 1 Argyreia nervosa 1 1 Carapa guianensis 1 1 Parthenium hysterophorus 1 1 Rhododendron anthopogon 1 1 Strobilanthes cusia 1 1 Dianthus superbus 1 1 Pyropolyporus fomentarius 1 1 Euphorbia hermentiana 1 1 Porteresia coarctata 1 1 2 Plant Chemical Count Activity Count Aerva lanata 1 1 Rivea corymbosa 1 1 Solanum mammosum 1 1 Juniperus horizontalis 1 1 Maytenus -

Conservation Gaps in Traditional Vegetables Native to Europe and Fennoscandia
agriculture Article Conservation Gaps in Traditional Vegetables Native to Europe and Fennoscandia Kauê de Sousa 1,2 and Svein Øivind Solberg 1,* 1 Department of Agricultural Sciences, Faculty of Applied Ecology, Agricultural Sciences and Biotechnology Inland Norway University of Applied Sciences, 2318 Hamar, Norway; [email protected] 2 Bioversity International, 00054 Rome, Italy * Correspondence: [email protected]; Tel.: +46-7354-01516 Received: 15 July 2020; Accepted: 3 August 2020; Published: 6 August 2020 Abstract: Vegetables are rich in vitamins and other micronutrients and are important crops for healthy diets and diversification of the food system, and many traditional (also termed underutilized or indigenous) species may play a role. The current study analyzed 35 vegetables with a European region of diversity with the effort to map the conservation status in Fennoscandia and beyond. We mapped georeferenced occurrences and current genebank holdings based on global databases and conducted conservation gaps analysis based on representativeness scores in situ and ex situ. Out of the 35 target species, 19 got at a high priority score for further conservation initiatives, while another 14 species got a medium priority score. We identified a pattern where traditional vegetables are poorly represented in genebank holdings. This corresponds well to a lack of attention in the scientific community measured in number of published papers. Considering the grand challenges ahead in terms of climate change, population growth and demand for sustainability, traditional vegetables deserve greater attention. Our contribution is to provide a basis for conservation priorities among the identified vegetables species native to Fennoscandia. Keywords: crop wild relatives; ecosystem services; ensemble models; genetic diversity; plant genetic resources; species distribution models 1.