The Carnot Cycle
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-
![Arxiv:2008.06405V1 [Physics.Ed-Ph] 14 Aug 2020 Fig.1 Shows Four Classical Cycles: (A) Carnot Cycle, (B) Stirling Cycle, (C) Otto Cycle and (D) Diesel Cycle](https://docslib.b-cdn.net/cover/0049/arxiv-2008-06405v1-physics-ed-ph-14-aug-2020-fig-1-shows-four-classical-cycles-a-carnot-cycle-b-stirling-cycle-c-otto-cycle-and-d-diesel-cycle-70049.webp)
Arxiv:2008.06405V1 [Physics.Ed-Ph] 14 Aug 2020 Fig.1 Shows Four Classical Cycles: (A) Carnot Cycle, (B) Stirling Cycle, (C) Otto Cycle and (D) Diesel Cycle
Investigating student understanding of heat engine: a case study of Stirling engine Lilin Zhu1 and Gang Xiang1, ∗ 1Department of Physics, Sichuan University, Chengdu 610064, China (Dated: August 17, 2020) We report on the study of student difficulties regarding heat engine in the context of Stirling cycle within upper-division undergraduate thermal physics course. An in-class test about a Stirling engine with a regenerator was taken by three classes, and the students were asked to perform one of the most basic activities—calculate the efficiency of the heat engine. Our data suggest that quite a few students have not developed a robust conceptual understanding of basic engineering knowledge of the heat engine, including the function of the regenerator and the influence of piston movements on the heat and work involved in the engine. Most notably, although the science error ratios of the three classes were similar (∼10%), the engineering error ratios of the three classes were high (above 50%), and the class that was given a simple tutorial of engineering knowledge of heat engine exhibited significantly smaller engineering error ratio by about 20% than the other two classes. In addition, both the written answers and post-test interviews show that most of the students can only associate Carnot’s theorem with Carnot cycle, but not with other reversible cycles working between two heat reservoirs, probably because no enough cycles except Carnot cycle were covered in the traditional Thermodynamics textbook. Our results suggest that both scientific and engineering knowledge are important and should be included in instructional approaches, especially in the Thermodynamics course taught in the countries and regions with a tradition of not paying much attention to experimental education or engineering training. -

Fuel Cells Versus Heat Engines: a Perspective of Thermodynamic and Production
Fuel Cells Versus Heat Engines: A Perspective of Thermodynamic and Production Efficiencies Introduction: Fuel Cells are being developed as a powering method which may be able to provide clean and efficient energy conversion from chemicals to work. An analysis of their real efficiencies and productivity vis. a vis. combustion engines is made in this report. The most common mode of transportation currently used is gasoline or diesel engine powered automobiles. These engines are broadly described as internal combustion engines, in that they develop mechanical work by the burning of fossil fuel derivatives and harnessing the resultant energy by allowing the hot combustion product gases to expand against a cylinder. This arrangement allows for the fuel heat release and the expansion work to be performed in the same location. This is in contrast to external combustion engines, in which the fuel heat release is performed separately from the gas expansion that allows for mechanical work generation (an example of such an engine is steam power, where fuel is used to heat a boiler, and the steam then drives a piston). The internal combustion engine has proven to be an affordable and effective means of generating mechanical work from a fuel. However, because the majority of these engines are powered by a hydrocarbon fossil fuel, there has been recent concern both about the continued availability of fossil fuels and the environmental effects caused by the combustion of these fuels. There has been much recent publicity regarding an alternate means of generating work; the hydrogen fuel cell. These fuel cells produce electric potential work through the electrochemical reaction of hydrogen and oxygen, with the reaction product being water. -

The Aircraft Propulsion the Aircraft Propulsion
THE AIRCRAFT PROPULSION Aircraft propulsion Contact: Ing. Miroslav Šplíchal, Ph.D. [email protected] Office: A1/0427 Aircraft propulsion Organization of the course Topics of the lectures: 1. History of AE, basic of thermodynamic of heat engines, 2-stroke and 4-stroke cycle 2. Basic parameters of piston engines, types of piston engines 3. Design of piston engines, crank mechanism, 4. Design of piston engines - auxiliary systems of piston engines, 5. Performance characteristics increase performance, propeller. 6. Turbine engines, introduction, input system, centrifugal compressor. 7. Turbine engines - axial compressor, combustion chamber. 8. Turbine engines – turbine, nozzles. 9. Turbine engines - increasing performance, construction of gas turbine engines, 10. Turbine engines - auxiliary systems, fuel-control system. 11. Turboprop engines, gearboxes, performance. 12. Maintenance of turbine engines 13. Ramjet engines and Rocket engines Aircraft propulsion Organization of the course Topics of the seminars: 1. Basic parameters of piston engine + presentation (1-7)- 3.10.2017 2. Parameters of centrifugal flow compressor + presentation(8-14) - 17.10.2017 3. Loading of turbine blade + presentation (15-21)- 31.10.2017 4. Jet engine cycle + presentation (22-28) - 14.11.2017 5. Presentation alternative date Seminar work: Aircraft engines presentation A short PowerPoint presentation, aprox. 10 minutes long. Content of presentation: - a brief history of the engine - the main innovation introduced by engine - engine drawing / cross-section - -

Carnot Cycles
CARNOT CYCLES Sadi Carnot was a French physicist who proposed an “ideal” cycle for a heat engine in 1824. Historical note – the idea of an ideal cycle came about because engineers were trying to develop a steam engine (a type of heat engine) where they could reject (waste) a minimal amount of heat. This would produce the best efficiency since η = 1 – (QL/QH). Carnot proposed that a cycle comprised of completely (internally and externally) reversible processes would give the maximum amount of net work for a given heat input, since the work done by a system in a reversible (ideal) process is always greater than that in an irreversible (real) process. THE CARNOT HEAT ENGINE CYCLE CONSISTS OF FOUR REVERSIBLE PROCESSES IN A SEQUENCE: 1 Æ 2: Reversible isothermal expansion. Heat transfer from HTR (+) and boundary work (+) occur in closed system 2 Æ 3: Reversible adiabatic expansion Work output (+), but no heat transfer 3 Æ 4: Reversible isothermal compression Heat transfer (-) and boundary work (-) occur in closed system 4 Æ 1: Reversible adiabatic compression Work input (-), but no heat transfer AND Wout >>> Win 1 P-V DIAGRAM FOR CARNOT HEAT ENGINE CYCLE P 1 2 4 3 Showing net work is POSITIVE. V A useful example of an isothermal expansion is boiling (vaporization) at a constant pressure in a device such as a piston-cylinder. Similarly, an example of an isothermal compression is condensation at a constant pressure in a piston-cylinder. Also, heat transfer can only occur in processes 1 Æ 2 and 3 Æ4. 1 Æ 2: since work is positive (expansion) and Δu is positive (e.g., boiling) then heat transfer is positive (input from HTR). -

Lecture 10. Heat Engines (Ch. 4)
Lecture 11. Heat Engines (Ch. 4) A heat engine – any device that is capable of converting thermal energy (heating) into mechanical energy (work). We will consider an important class of such devices whose operation is cyclic. Heating – the transfer of energy to a system by thermal contact with a reservoir. Work – the transfer of energy to a system by a change in the external parameters (V, el.-mag. and grav. fields, etc.). The main question we want to address: what are the limitations imposed by thermodynamic on the performance of heat engines? Perpetual Motion Machines are Impossible Perpetual Motion Machines of the first type – these designs seek to violation of the First Law create the energy required for their (energy conservation) operation out of nothing. Perpetual Motion Machines of the second type - these designs extract the energy required for their operation violation of the Second in a manner that decreases the entropy Law of an isolated system. hot reservoir Word of caution: for non-cyclic processes, T H 100% of heat can be transformed into work without violating the Second Law. heat Example: an ideal gas expands isothermally work being in thermal contact with a hot reservoir. Since U = const at T = const, all heat has been transformed into work. impossible cyclic heat engine Fundamental Difference between Heating and Work - is the difference in the entropy transfer! Transferring purely mechanical energy to or from a system does not (necessarily) change its entropy: ΔS = 0 for reversible processes. For this reason, all forms of work are thermodynamically equivalent to each other - they are freely convertible into each other and, in particular, into mechanical work. -

Carnot Heat Engine Efficiency with a Paramagnetic Gas
Carnot heat engine efficiency with a paramagnetic gas Manuel Malaver1,2,3 1 Bijective Physics Institute, Bijective Physics Group, Idrija, Slovenia 2 Entro-π Fluid Research Group. 3 Maritime University of the Caribbean, Catia la Mar, Venezuela. *Corresponding author (Email: [email protected]) Abstract – Considering ideal paramagnetic medium, in this paper we deduced an expression for the thermal efficiency of Carnot heat engine with a paramagnetic gas as working substance. We found that the efficiency depends on the limits of maximum and minimal temperature imposed on the Carnot cycle, as in the ideal gas, photon gas and variable Chaplygin gas. Keywords – Carnot heat engine, ideal paramagnetic medium, thermal efficiency, paramagnetic gas. 1. Introduction The magnetism is a type of associated physical phenomena with the orbital and spin motions of electrons and the interaction of electrons with each other (Ahmed et al., 2019). The electric currents and the atomic magnetic moment of elementary particles generate magnetic fields that can act on other currents and magnetic materials. The most common effect occurs in ferromagnetic solids which is possible when atoms are rearrange in such a way that magnetic atomic moments can interact to align parallel to each other (Eisberg and Resnick, 1991; Chikazumi, 2009). In quantum mechanics, the ferromagnetism can be described as a parallel alignment of magnetic moments what depends of the interaction between neighbouring moments (Feynman et al., 1963). Although ferromagnetism is the cause of many frequently observed magnetism effects, not all materials respond equally to magnetic fields because the interaction between magnetic atomic moments is very weak while in other materials this interaction is stronger (Ahmed et al., 2019). -

The Carnot Cycle, Reversibility and Entropy
entropy Article The Carnot Cycle, Reversibility and Entropy David Sands Department of Physics and Mathematics, University of Hull, Hull HU6 7RX, UK; [email protected] Abstract: The Carnot cycle and the attendant notions of reversibility and entropy are examined. It is shown how the modern view of these concepts still corresponds to the ideas Clausius laid down in the nineteenth century. As such, they reflect the outmoded idea, current at the time, that heat is motion. It is shown how this view of heat led Clausius to develop the entropy of a body based on the work that could be performed in a reversible process rather than the work that is actually performed in an irreversible process. In consequence, Clausius built into entropy a conflict with energy conservation, which is concerned with actual changes in energy. In this paper, reversibility and irreversibility are investigated by means of a macroscopic formulation of internal mechanisms of damping based on rate equations for the distribution of energy within a gas. It is shown that work processes involving a step change in external pressure, however small, are intrinsically irreversible. However, under idealised conditions of zero damping the gas inside a piston expands and traces out a trajectory through the space of equilibrium states. Therefore, the entropy change due to heat flow from the reservoir matches the entropy change of the equilibrium states. This trajectory can be traced out in reverse as the piston reverses direction, but if the external conditions are adjusted appropriately, the gas can be made to trace out a Carnot cycle in P-V space. -

Lecture 11 Second Law of Thermodynamics
LECTURE 11 SECOND LAW OF THERMODYNAMICS Lecture instructor: Kazumi Tolich Lecture 11 2 ¨ Reading chapter 18.5 to 18.10 ¤ The second law of thermodynamics ¤ Heat engines ¤ Refrigerators ¤ Air conditions ¤ Heat pumps ¤ Entropy Second law of thermodynamics 3 ¨ The second law of thermodynamics states: Thermal energy flows spontaneously from higher to lower temperature. Heat engines are always less than 100% efficient at using thermal energy to do work. The total entropy of all the participants in any physical process cannot decrease during that process. Heat engines 4 ¨ Heat engines depend on the spontaneous heat flow from hot to cold to do work. ¨ In each cycle, the hot reservoir supplies heat �" to the engine which does work �and exhausts heat �$ to the cold reservoir. � = �" − �$ ¨ The energy efficiency of a heat engine is given by � � = �" Carnot’s theorem and maximum efficiency 5 ¨ The maximum-efficiency heat engine is described in Carnot’s theorem: If an engine operating between two constant-temperature reservoirs is to have maximum efficiency, it must be an engine in which all processes are reversible. In addition, all reversible engines operating between the same two temperatures, �$ and �", have the same efficiency. ¨ This is an idealization; no real engine can be perfectly reversible. ¨ The maximum efficiency of a heat engine (Carnot engine) can be written as: �$ �*+, = 1 − �" Quiz: 1 6 ¨ Consider the heat engine at right. �. denotes the heat extracted from the hot reservoir, and �/ denotes the heat exhausted to the cold reservoir in one cycle. What is the work done by this engine in one cycle in Joules? Quiz: 11-1 answer 7 ¨ 4000 J ¨ � = �. -

The Carnot Cycle Ray Fu
The Carnot Cycle Ray Fu The Carnot Cycle We will investigate some properties of the Carnot cycle and explore the subtleties of Carnot's theorem. 1. Recall that the Carnot cycle consists of the following four reversible steps, in order: (a) isothermal expansion at TH (b) isentropic expansion at Smax (c) isothermal compression at TC (d) isentropic compression at Smin TH and TC represent the temperatures of the hot and cold thermal reservoirs; Smin and Smax represent the minimum and maximum entropy reached by the working fluid in the Carnot cycle. Draw the Carnot cycle on a TS plot and label each step of the cycle, as well as TH , TC , Smin, and Smax. 2. On the same plot, sketch the cycle that results when steps (a) and (c) are respectively replaced with irreversible isothermal expansion and compression, assuming that the resulting cycle operates between the same two entropies Smin and Smax. We will prove geometrically that the Carnot cycle is the most efficient thermodynamic cycle working between the range of temperatures bounded by TH and TC . To this end, consider an arbitrary reversible thermody- namic cycle C with working fluid having maximum and minimum temperatures TH and TC , and maximum and minimum entropies Smax and Smin. It is sufficient to consider reversible cycles, for irreversible cycles must have lower efficiencies by Clausius's inequality. 3. Define two areas A and B on the corresponding TS plot for C. A is the area enclosed by C, whereas B is the area bounded above by the bottom of C, bounded below by the S-axis, and bounded to left and right by the lines S = Smin and S = Smax. -

Thermodynamics Cycle Analysis and Numerical Modeling of Thermoelastic Cooling Systems
international journal of refrigeration 56 (2015) 65e80 Available online at www.sciencedirect.com ScienceDirect www.iifiir.org journal homepage: www.elsevier.com/locate/ijrefrig Thermodynamics cycle analysis and numerical modeling of thermoelastic cooling systems Suxin Qian, Jiazhen Ling, Yunho Hwang*, Reinhard Radermacher, Ichiro Takeuchi Center for Environmental Energy Engineering, Department of Mechanical Engineering, University of Maryland, 4164 Glenn L. Martin Hall Bldg., College Park, MD 20742, USA article info abstract Article history: To avoid global warming potential gases emission from vapor compression air- Received 3 October 2014 conditioners and water chillers, alternative cooling technologies have recently garnered Received in revised form more and more attentions. Thermoelastic cooling is among one of the alternative candi- 3 March 2015 dates, and have demonstrated promising performance improvement potential on the Accepted 2 April 2015 material level. However, a thermoelastic cooling system integrated with heat transfer fluid Available online 14 April 2015 loops have not been studied yet. This paper intends to bridge such a gap by introducing the single-stage cycle design options at the beginning. An analytical coefficient of performance Keywords: (COP) equation was then derived for one of the options using reverse Brayton cycle design. Shape memory alloy The equation provides physical insights on how the system performance behaves under Elastocaloric different conditions. The performance of the same thermoelastic cooling cycle using NiTi Efficiency alloy was then evaluated based on a dynamic model developed in this study. It was found Nitinol that the system COP was 1.7 for a baseline case considering both driving motor and Solid-state cooling parasitic pump power consumptions, while COP ranged from 5.2 to 7.7 when estimated with future improvements. -
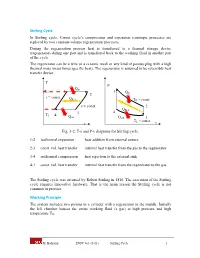
Stirling Cycle Working Principle
Stirling Cycle In Stirling cycle, Carnot cycle’s compression and expansion isentropic processes are replaced by two constant-volume regeneration processes. During the regeneration process heat is transferred to a thermal storage device (regenerator) during one part and is transferred back to the working fluid in another part of the cycle. The regenerator can be a wire or a ceramic mesh or any kind of porous plug with a high thermal mass (mass times specific heat). The regenerator is assumed to be reversible heat transfer device. T P Qin TH 1 1 Q 2 in v = const. Q Reg. TH = const. v = const. 2 QReg. 3 4 TL 4 Qout Qout 3 T = const. s L v Fig. 3-2: T-s and P-v diagrams for Stirling cycle. 1-2 isothermal expansion heat addition from external source 2-3 const. vol. heat transfer internal heat transfer from the gas to the regenerator 3-4 isothermal compression heat rejection to the external sink 4-1 const. vol. heat transfer internal heat transfer from the regenerator to the gas The Stirling cycle was invented by Robert Stirling in 1816. The execution of the Stirling cycle requires innovative hardware. That is the main reason the Stirling cycle is not common in practice. Working Principle The system includes two pistons in a cylinder with a regenerator in the middle. Initially the left chamber houses the entire working fluid (a gas) at high pressure and high temperature TH. M. Bahrami ENSC 461 (S 11) Stirling Cycle 1 TH TL QH State 1 Regenerator Fig 3-3a: 1-2, isothermal heat transfer to the gas at TH from external source. -

Exergo-Sustainability Analysis and Ecological Function of a Simple Gas Turbine Aero-Engine
Journal of Thermal Engineering, Vol. 4, No. 4, Special Issue 8, pp. 2083-2095, June, 2018 Yildiz Technical University Press, Istanbul, Turkey EXERGO-SUSTAINABILITY ANALYSIS AND ECOLOGICAL FUNCTION OF A SIMPLE GAS TURBINE AERO-ENGINE Y. Şöhret1,* ABSTRACT Nowadays, many environmental issues are of concern as a result of conventional energy resources utilization in addition to a rise in energy costs dependent on the rapid consumption of resources. Therefore, sustainability is an important term for the utilization of energy resources. The aviation industry is known to be responsible for 3% of total CO2 emissions concerning global warming. This forces us to investigate the aviation industry, specifically gas turbine aero-engines. Gas turbine aero-engines, working according to the principles of thermodynamics, similar to other energy conversion and generation systems can be evaluated using the first and second laws of thermodynamics. Integrated employment of the first and second laws of thermodynamics, namely exergy analysis, is an effective method for performance evaluation. Additionally, exergo-sustainability also yields beneficial results. In the framework of the current paper, ecological function is defined for a simple gas turbine aero-engine, while exergo-sustainability assessment methodology is also explained. Exergy efficiency of the compressor, combustor, gas turbine and nozzle, as components of a gas turbine aero-engine, is found to be 91.58%, 57.41%, 97.96%, and 61.25%, respectively. On the other hand, the sustainability measures of the evaluated gas turbine aero-engine in order of exergy efficiency, waste exergy ratio, recoverable exergy rate, exergy destruction factor, environmental effect factor and sustainability index are calculated to be 0.28, 0.71, 0.00, 0.69, 2.45, and 0.40, respectively whereas the ecological function is found to be -8732.21 kW.