Fuel Cells Versus Heat Engines: a Perspective of Thermodynamic and Production
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Physics 170 - Thermodynamic Lecture 40
Physics 170 - Thermodynamic Lecture 40 ! The second law of thermodynamic 1 The Second Law of Thermodynamics and Entropy There are several diferent forms of the second law of thermodynamics: ! 1. In a thermal cycle, heat energy cannot be completely transformed into mechanical work. ! 2. It is impossible to construct an operational perpetual-motion machine. ! 3. It’s impossible for any process to have as its sole result the transfer of heat from a cooler to a hotter body ! 4. Heat flows naturally from a hot object to a cold object; heat will not flow spontaneously from a cold object to a hot object. ! ! Heat Engines and Thermal Pumps A heat engine converts heat energy into work. According to the second law of thermodynamics, however, it cannot convert *all* of the heat energy supplied to it into work. Basic heat engine: hot reservoir, cold reservoir, and a machine to convert heat energy into work. Heat Engines and Thermal Pumps 4 Heat Engines and Thermal Pumps This is a simplified diagram of a heat engine, along with its thermal cycle. Heat Engines and Thermal Pumps An important quantity characterizing a heat engine is the net work it does when going through an entire cycle. Heat Engines and Thermal Pumps Heat Engines and Thermal Pumps Thermal efciency of a heat engine: ! ! ! ! ! ! From the first law, it follows: Heat Engines and Thermal Pumps Yet another restatement of the second law of thermodynamics: No cyclic heat engine can convert its heat input completely to work. Heat Engines and Thermal Pumps A thermal pump is the opposite of a heat engine: it transfers heat energy from a cold reservoir to a hot one. -

Physics 100 Lecture 7
2 Physics 100 Lecture 7 Heat Engines and the 2nd Law of Thermodynamics February 12, 2018 3 Thermal Convection Warm fluid is less dense and rises while cool fluid sinks Resulting circulation efficiently transports thermal energy 4 COLD Convection HOT Turbulent motion of glycerol in a container heated from below and cooled from above. The bright lines show regions of rapid temperature variation. The fluid contains many "plumes," especially near the walls. The plumes can be identified as mushroom-shaped objects with heat flowing through the "stalk" and spreading in the "cap." The hot plumes tend to rise with their caps on top; falling, cold plumes are cap-down. All this plume activity is carried along in an overall counterclockwise "wind" caused by convection. Note the thermometer coming down from the top of the cell. Figure adapted from J. Zhang, S. Childress, A. Libchaber, Phys. Fluids 9, 1034 (1997). See detailed discussion in Kadanoff, L. P., Physics Today 54, 34 (August 2001). 5 The temperature of land changes more quickly than the nearby ocean. Thus convective “sea breezes” blow ____ during the day and ____ during the night. A. onshore … onshore B. onshore … offshore C. offshore … onshore D. offshore … offshore 6 The temperature of land changes more quickly than the nearby ocean. Thus convective “sea breezes” blow ____ during the day and ____ during the night. A. onshore … onshore B.onshore … offshore C.offshore … onshore D.offshore … offshore 7 Thermal radiation Any object whose temperature is above zero Kelvin emits energy in the form of electromagnetic radiation Objects both absorb and emit EM radiation continuously, and this phenomenon helps determine the object’s equilibrium temperature 8 The electromagnetic spectrum 9 Thermal radiation We’ll examine this concept some more in chapter 6 10 Why does the Earth cool more quickly on clear nights than it does on cloudy nights? A. -

Recording and Evaluating the Pv Diagram with CASSY
LD Heat Physics Thermodynamic cycle Leaflets P2.6.2.4 Hot-air engine: quantitative experiments The hot-air engine as a heat engine: Recording and evaluating the pV diagram with CASSY Objects of the experiment Recording the pV diagram for different heating voltages. Determining the mechanical work per revolution from the enclosed area. Principles The cycle of a heat engine is frequently represented as a closed curve in a pV diagram (p: pressure, V: volume). Here the mechanical work taken from the system is given by the en- closed area: W = − ͛ p ⋅ dV (I) The cycle of the hot-air engine is often described in an idealised form as a Stirling cycle (see Fig. 1), i.e., a succession of isochoric heating (a), isothermal expansion (b), isochoric cooling (c) and isothermal compression (d). This description, however, is a rough approximation because the working piston moves sinusoidally and therefore an isochoric change of state cannot be expected. In this experiment, the pV diagram is recorded with the computer-assisted data acquisition system CASSY for comparison with the real behaviour of the hot-air engine. A pressure sensor measures the pressure p in the cylinder and a displacement sensor measures the position s of the working piston, from which the volume V is calculated. The measured values are immediately displayed on the monitor in a pV diagram. Fig. 1 pV diagram of the Stirling cycle 0210-Wei 1 P2.6.2.4 LD Physics Leaflets Setup Apparatus The experimental setup is illustrated in Fig. 2. 1 hot-air engine . 388 182 1 U-core with yoke . -

Measuring the Steady State Thermal Efficiency of a Heating System
Measuring the Steady State Thermal Efficiency of a Heating System. Last week you had a tour of “Colby Power and Light” and had a chance to see Gus Libby’s really cool, campus-scale, multi-million dollar calorimeter. This week we will use lab-scale calorimeters to measure the heat and carbon content of different fuels (methane, propane, butane, and gasoline). Our simple calorimeters were designed by Whitney King and Chuck Jones, Science Division Instrument Wizard, to simulate the highly efficient HTP Versa Flame fire-tube boiler. The lab has three parts: 1) Calibrate the calorimeter 2) Measure the heat content of different fuels 3) Compare the measured heat content to the calculated heat content 1) Calibrate the Calorimeter. You lab instructor will demonstrate the assembly of the laboratory- scale fire-tube calorimeter. The heat transferred from the burning fuel to the calorimeter can be determined from the mass of the object being heated, the heat capacity of the object and the change in temperature. � = ���� ∗ �� ∗ ∆� We will begin the experiment by adding a known amount of heat to our calorimeters from a coffee cup heater. The heater is a simple resistor that converts electrical energy into heat. The heater has a resistance of 47.5 ohms and is plugged into a 120 V outlet. Recalling your electricity basics, we know that V=IR where V is the voltage in Figure 1. HTP Versa Flame Fire Tube volts, I is the current in amps, and R is the Boiler. Brown Tubes carry the hot resistance in ohms. The power, P, consumed by combustion gasses inside of the the heater is volts times current, water tank where they condense releasing the maximum available 2 heat. -

Thermodynamics of Power Generation
THERMAL MACHINES AND HEAT ENGINES Thermal machines ......................................................................................................................................... 1 The heat engine ......................................................................................................................................... 2 What it is ............................................................................................................................................... 2 What it is for ......................................................................................................................................... 2 Thermal aspects of heat engines ........................................................................................................... 3 Carnot cycle .............................................................................................................................................. 3 Gas power cycles ...................................................................................................................................... 4 Otto cycle .............................................................................................................................................. 5 Diesel cycle ........................................................................................................................................... 8 Brayton cycle ..................................................................................................................................... -

Power Plant Steam Cycle Theory - R.A
THERMAL POWER PLANTS – Vol. I - Power Plant Steam Cycle Theory - R.A. Chaplin POWER PLANT STEAM CYCLE THEORY R.A. Chaplin Department of Chemical Engineering, University of New Brunswick, Canada Keywords: Steam Turbines, Carnot Cycle, Rankine Cycle, Superheating, Reheating, Feedwater Heating. Contents 1. Cycle Efficiencies 1.1. Introduction 1.2. Carnot Cycle 1.3. Simple Rankine Cycles 1.4. Complex Rankine Cycles 2. Turbine Expansion Lines 2.1. T-s and h-s Diagrams 2.2. Turbine Efficiency 2.3. Turbine Configuration 2.4. Part Load Operation Glossary Bibliography Biographical Sketch Summary The Carnot cycle is an ideal thermodynamic cycle based on the laws of thermodynamics. It indicates the maximum efficiency of a heat engine when operating between given temperatures of heat acceptance and heat rejection. The Rankine cycle is also an ideal cycle operating between two temperature limits but it is based on the principle of receiving heat by evaporation and rejecting heat by condensation. The working fluid is water-steam. In steam driven thermal power plants this basic cycle is modified by incorporating superheating and reheating to improve the performance of the turbine. UNESCO – EOLSS The Rankine cycle with its modifications suggests the best efficiency that can be obtained from this two phaseSAMPLE thermodynamic cycle wh enCHAPTERS operating under given temperature limits but its efficiency is less than that of the Carnot cycle since some heat is added at a lower temperature. The efficiency of the Rankine cycle can be improved by regenerative feedwater heating where some steam is taken from the turbine during the expansion process and used to preheat the feedwater before it is evaporated in the boiler. -

The Aircraft Propulsion the Aircraft Propulsion
THE AIRCRAFT PROPULSION Aircraft propulsion Contact: Ing. Miroslav Šplíchal, Ph.D. [email protected] Office: A1/0427 Aircraft propulsion Organization of the course Topics of the lectures: 1. History of AE, basic of thermodynamic of heat engines, 2-stroke and 4-stroke cycle 2. Basic parameters of piston engines, types of piston engines 3. Design of piston engines, crank mechanism, 4. Design of piston engines - auxiliary systems of piston engines, 5. Performance characteristics increase performance, propeller. 6. Turbine engines, introduction, input system, centrifugal compressor. 7. Turbine engines - axial compressor, combustion chamber. 8. Turbine engines – turbine, nozzles. 9. Turbine engines - increasing performance, construction of gas turbine engines, 10. Turbine engines - auxiliary systems, fuel-control system. 11. Turboprop engines, gearboxes, performance. 12. Maintenance of turbine engines 13. Ramjet engines and Rocket engines Aircraft propulsion Organization of the course Topics of the seminars: 1. Basic parameters of piston engine + presentation (1-7)- 3.10.2017 2. Parameters of centrifugal flow compressor + presentation(8-14) - 17.10.2017 3. Loading of turbine blade + presentation (15-21)- 31.10.2017 4. Jet engine cycle + presentation (22-28) - 14.11.2017 5. Presentation alternative date Seminar work: Aircraft engines presentation A short PowerPoint presentation, aprox. 10 minutes long. Content of presentation: - a brief history of the engine - the main innovation introduced by engine - engine drawing / cross-section - -

25 Kw Low-Temperature Stirling Engine for Heat Recovery, Solar, and Biomass Applications
25 kW Low-Temperature Stirling Engine for Heat Recovery, Solar, and Biomass Applications Lee SMITHa, Brian NUELa, Samuel P WEAVERa,*, Stefan BERKOWERa, Samuel C WEAVERb, Bill GROSSc aCool Energy, Inc, 5541 Central Avenue, Boulder CO 80301 bProton Power, Inc, 487 Sam Rayburn Parkway, Lenoir City TN 37771 cIdealab, 130 W. Union St, Pasadena CA 91103 *Corresponding author: [email protected] Keywords: Stirling engine, waste heat recovery, concentrating solar power, biomass power generation, low-temperature power generation, distributed generation ABSTRACT This paper covers the design, performance optimization, build, and test of a 25 kW Stirling engine that has demonstrated > 60% of the Carnot limit for thermal to electrical conversion efficiency at test conditions of 329 °C hot side temperature and 19 °C rejection temperature. These results were enabled by an engine design and construction that has minimal pressure drop in the gas flow path, thermal conduction losses that are limited by design, and which employs a novel rotary drive mechanism. Features of this engine design include high-surface- area heat exchangers, nitrogen as the working fluid, a single-acting alpha configuration, and a design target for operation between 150 °C and 400 °C. 1 1. INTRODUCTION Since 2006, Cool Energy, Inc. (CEI) has designed, fabricated, and tested five generations of low-temperature (150 °C to 400 °C) Stirling engines that drive internally integrated electric alternators. The fifth generation of engine built by Cool Energy is rated at 25 kW of electrical power output, and is trade-named the ThermoHeart® Engine. Sources of low-to-medium temperature thermal energy, such as internal combustion engine exhaust, industrial waste heat, flared gas, and small-scale solar heat, have relatively few methods available for conversion into more valuable electrical energy, and the thermal energy is usually wasted. -

Carnot Cycles
CARNOT CYCLES Sadi Carnot was a French physicist who proposed an “ideal” cycle for a heat engine in 1824. Historical note – the idea of an ideal cycle came about because engineers were trying to develop a steam engine (a type of heat engine) where they could reject (waste) a minimal amount of heat. This would produce the best efficiency since η = 1 – (QL/QH). Carnot proposed that a cycle comprised of completely (internally and externally) reversible processes would give the maximum amount of net work for a given heat input, since the work done by a system in a reversible (ideal) process is always greater than that in an irreversible (real) process. THE CARNOT HEAT ENGINE CYCLE CONSISTS OF FOUR REVERSIBLE PROCESSES IN A SEQUENCE: 1 Æ 2: Reversible isothermal expansion. Heat transfer from HTR (+) and boundary work (+) occur in closed system 2 Æ 3: Reversible adiabatic expansion Work output (+), but no heat transfer 3 Æ 4: Reversible isothermal compression Heat transfer (-) and boundary work (-) occur in closed system 4 Æ 1: Reversible adiabatic compression Work input (-), but no heat transfer AND Wout >>> Win 1 P-V DIAGRAM FOR CARNOT HEAT ENGINE CYCLE P 1 2 4 3 Showing net work is POSITIVE. V A useful example of an isothermal expansion is boiling (vaporization) at a constant pressure in a device such as a piston-cylinder. Similarly, an example of an isothermal compression is condensation at a constant pressure in a piston-cylinder. Also, heat transfer can only occur in processes 1 Æ 2 and 3 Æ4. 1 Æ 2: since work is positive (expansion) and Δu is positive (e.g., boiling) then heat transfer is positive (input from HTR). -

Lecture 10. Heat Engines (Ch. 4)
Lecture 11. Heat Engines (Ch. 4) A heat engine – any device that is capable of converting thermal energy (heating) into mechanical energy (work). We will consider an important class of such devices whose operation is cyclic. Heating – the transfer of energy to a system by thermal contact with a reservoir. Work – the transfer of energy to a system by a change in the external parameters (V, el.-mag. and grav. fields, etc.). The main question we want to address: what are the limitations imposed by thermodynamic on the performance of heat engines? Perpetual Motion Machines are Impossible Perpetual Motion Machines of the first type – these designs seek to violation of the First Law create the energy required for their (energy conservation) operation out of nothing. Perpetual Motion Machines of the second type - these designs extract the energy required for their operation violation of the Second in a manner that decreases the entropy Law of an isolated system. hot reservoir Word of caution: for non-cyclic processes, T H 100% of heat can be transformed into work without violating the Second Law. heat Example: an ideal gas expands isothermally work being in thermal contact with a hot reservoir. Since U = const at T = const, all heat has been transformed into work. impossible cyclic heat engine Fundamental Difference between Heating and Work - is the difference in the entropy transfer! Transferring purely mechanical energy to or from a system does not (necessarily) change its entropy: ΔS = 0 for reversible processes. For this reason, all forms of work are thermodynamically equivalent to each other - they are freely convertible into each other and, in particular, into mechanical work. -

Carnot Heat Engine Efficiency with a Paramagnetic Gas
Carnot heat engine efficiency with a paramagnetic gas Manuel Malaver1,2,3 1 Bijective Physics Institute, Bijective Physics Group, Idrija, Slovenia 2 Entro-π Fluid Research Group. 3 Maritime University of the Caribbean, Catia la Mar, Venezuela. *Corresponding author (Email: [email protected]) Abstract – Considering ideal paramagnetic medium, in this paper we deduced an expression for the thermal efficiency of Carnot heat engine with a paramagnetic gas as working substance. We found that the efficiency depends on the limits of maximum and minimal temperature imposed on the Carnot cycle, as in the ideal gas, photon gas and variable Chaplygin gas. Keywords – Carnot heat engine, ideal paramagnetic medium, thermal efficiency, paramagnetic gas. 1. Introduction The magnetism is a type of associated physical phenomena with the orbital and spin motions of electrons and the interaction of electrons with each other (Ahmed et al., 2019). The electric currents and the atomic magnetic moment of elementary particles generate magnetic fields that can act on other currents and magnetic materials. The most common effect occurs in ferromagnetic solids which is possible when atoms are rearrange in such a way that magnetic atomic moments can interact to align parallel to each other (Eisberg and Resnick, 1991; Chikazumi, 2009). In quantum mechanics, the ferromagnetism can be described as a parallel alignment of magnetic moments what depends of the interaction between neighbouring moments (Feynman et al., 1963). Although ferromagnetism is the cause of many frequently observed magnetism effects, not all materials respond equally to magnetic fields because the interaction between magnetic atomic moments is very weak while in other materials this interaction is stronger (Ahmed et al., 2019). -
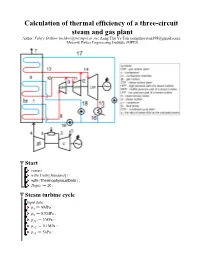
Calculation of Thermal Efficiency of a Three-Circuit Steam and Gas Plant
Calculation of thermal efficiency of a three-circuit steam and gas plant Author: Valery Ochkov ([email protected]), Aung Thu Ya Tun ([email protected]), Moscow Power Engineering Institute (MPEI) Start > > > > Steam turbine cycle Input data: > > > > > > > > > > > > > > Functions and procedures The specific enthalpy of the steam entering the turbine HPP: > (2.1) The specific enthalpy of steam at outlet of the turbine HPP: > (2.2) Specific work of HPP steam turbine: > (2.3) The temperature of the steam at the outlet of the HPP turbine: > (2.4) The specific enthalpy of steam at the point 10: > (2.5) > (2.6) The balance of the mixing streams at points 9 and 10: > (2.7) The temperature of steam at the inlet to the MPP of the turbine: > (2.8) > (2.8) > (2.9) > Specific enthalpy of water vapor at the inlet to the MPP of the turbine: > (2.10) Specific enthalpy of steam at the outlet of the turbine MPP: > (2.11) C : > (2.12) The temperature of the steam at the outlet of the MPP of the turbine: > (2.13) The specific enthalpy of steam at point 13: > (2.14) > (2.15) Balance when mixing streams at points 12 and 13: > (2.16) The temperature of steam at the inlet to the LPP of the turbine: > (2.17) > (2.18) The specific enthalpy of steam at outlet of the turbine LPP: > (2.19) Specific LPP operation of a steam turbine: > (2.8) > (2.20) The degree of dryness of the steam at the outlet of the turbine LPP: > (2.21) Condensate temperature: > (2.22) Specific enthalpy of condensate: > (2.23) The specific enthalpy of the feed water high pressure