Thermodynamics of Power Generation
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Constant Volume Combustion Cycle for IC Engines
Constant Volume Combustion Cycle for IC Engines Jovan Dorić Assistant This paper presents an analysis of the internal combustion engine cycle in University of Novi Sad Faculty of Technical Sciences cases when the new unconventional piston motion law is used. The main goal of the presented unconventional piston motion law is to make a Ivan Klinar realization of combustion during constant volume in an engine’s cylinder. Full Professor The obtained results are shown in PV (pressure-volume) diagrams of University of Novi Sad Faculty of Technical Sciences standard and new engine cycles. The emphasis is placed on the shortcomings of the real cycle in IC engines as well as policies that would Marko Dorić contribute to reducing these disadvantages. For this paper, volumetric PhD student efficiency, pressure and temperature curves for standard and new piston University of Novi Sad Faculty of Agriculture motion law have been calculated. Also, the results of improved efficiency and power are presented. Keywords: constant volume combustion, IC engine, kinematics. 1. INTRODUCTION of an internal combustion engine, whose combustion is so rapid that the piston does not move during the Internal combustion engine simulation modelling has combustion process, and thus combustion is assumed to long been established as an effective tool for studying take place at constant volume [6]. Although in engine performance and contributing to evaluation and theoretical terms heat addition takes place at constant new developments. Thermodynamic models of the real volume, in real engine cycle heat addition at constant engine cycle have served as effective tools for complete volume can not be performed. -

Steady State and Transient Efficiencies of A
STEADY STATE AND TRANSIENT EFFICIENCIES OF A FOUR CYLINDER DIRECT INJECTION DIESEL ENGINE FOR IMPLEMENTATION IN A HYBRID ELECTRIC VEHICLE A Thesis Presented to The Graduate Faculty of The University of Akron In Partial Fulfillment of the Requirements for the Degree Masters of Science Charles Van Horn August, 2006 STEADY STATE AND TRANSIENT EFFICIENCIES OF A FOUR CYLINDER DIRECT INJECTION DIESEL ENGINE FOR IMPLEMENTATION IN A HYBRID ELECTRIC VEHICLE Charles Van Horn Thesis Approved: Accepted: Advisor Department Chair Dr. Scott Sawyer Dr. Celal Batur Faculty Reader Dean of the College Dr. Richard Gross Dr. George K. Haritos Faculty Reader Dean of the Graduate School Dr. Iqbal Husain Dr. George R. Newkome Date ii ABSTRACT The efficiencies of a four cylinder direct injection diesel engine have been investigated for the implementation in a hybrid electric vehicle (HEV). The engine was cycled through various operating points depending on the power and torque requirements for the HEV. The selected engine for the HEV is a 2005 Volkswagen 1.9L diesel engine. The 2005 Volkswagen 1.9L diesel engine was tested to develop the steady-state engine efficiencies and to evaluate the transient effects on these efficiencies. The peak torque and power curves were developed using a water brake dynamometer. Once these curves were obtained steady-state testing at various engine speeds and powers was conducted to determine engine efficiencies. Transient operation of the engine was also explored using partial throttle and variable throttle testing. The transient efficiency was compared to the steady-state efficiencies and showed a decrease from the steady- state values. -

Benchmarking a 2018 Toyota Camry 2.5L Atkinson Cycle Engine With
Downloaded from SAE International by John Kargul, Thursday, April 04, 2019 2019-01-0249 Published 02 Apr 2019 Benchmarking a 2018 Toyota Camry 2.5-Liter INTERNATIONAL. Atkinson Cycle Engine with Cooled-EGR John Kargul, Mark Stuhldreher, Daniel Barba, Charles Schenk, Stanislav Bohac, Joseph McDonald, and Paul Dekraker US Environmental Protection Agency Josh Alden Southwest Research Institute Citation: Kargul, J., Stuhldreher, M., Barba, D., Schenk, C. et al., “Benchmarking a 2018 Toyota Camry 2.5-Liter Atkinson Cycle Engine with Cooled-EGR,” SAE Technical Paper 2019-01-0249, 2019, doi:10.4271/2019-01-0249. Abstract idle, low, medium, and high load engine operation. Motoring s part of the U.S. Environmental Protection Agency’s torque, wide open throttle (WOT) torque and fuel consumption (EPA’s) continuing assessment of advanced light-duty are measured during transient operation using both EPA Tier Aautomotive technologies in support of regulatory and 2 and Tier 3 test fuels. Te design and performance of this 2018 compliance programs, a 2018 Toyota Camry A25A-FKS 2.5-liter engine is described and compared to Toyota’s published 4-cylinder, 2.5-liter, naturally aspirated, Atkinson Cycle engine data and to EPA’s previous projections of the efciency of an with cooled exhaust gas recirculation (cEGR) was bench- Atkinson Cycle engine with cEGR. The Brake Thermal marked. Te engine was tested on an engine dynamometer Efciency (BTE) map for the Toyota A25A-FKS engine shows with and without its 8-speed automatic transmission, and with a peak efciency near 40 percent, which is the highest value of the engine wiring harness tethered to a complete vehicle parked any publicly available map for a non-hybrid production gasoline outside of the test cell. -

Physics 170 - Thermodynamic Lecture 40
Physics 170 - Thermodynamic Lecture 40 ! The second law of thermodynamic 1 The Second Law of Thermodynamics and Entropy There are several diferent forms of the second law of thermodynamics: ! 1. In a thermal cycle, heat energy cannot be completely transformed into mechanical work. ! 2. It is impossible to construct an operational perpetual-motion machine. ! 3. It’s impossible for any process to have as its sole result the transfer of heat from a cooler to a hotter body ! 4. Heat flows naturally from a hot object to a cold object; heat will not flow spontaneously from a cold object to a hot object. ! ! Heat Engines and Thermal Pumps A heat engine converts heat energy into work. According to the second law of thermodynamics, however, it cannot convert *all* of the heat energy supplied to it into work. Basic heat engine: hot reservoir, cold reservoir, and a machine to convert heat energy into work. Heat Engines and Thermal Pumps 4 Heat Engines and Thermal Pumps This is a simplified diagram of a heat engine, along with its thermal cycle. Heat Engines and Thermal Pumps An important quantity characterizing a heat engine is the net work it does when going through an entire cycle. Heat Engines and Thermal Pumps Heat Engines and Thermal Pumps Thermal efciency of a heat engine: ! ! ! ! ! ! From the first law, it follows: Heat Engines and Thermal Pumps Yet another restatement of the second law of thermodynamics: No cyclic heat engine can convert its heat input completely to work. Heat Engines and Thermal Pumps A thermal pump is the opposite of a heat engine: it transfers heat energy from a cold reservoir to a hot one. -

Physics 100 Lecture 7
2 Physics 100 Lecture 7 Heat Engines and the 2nd Law of Thermodynamics February 12, 2018 3 Thermal Convection Warm fluid is less dense and rises while cool fluid sinks Resulting circulation efficiently transports thermal energy 4 COLD Convection HOT Turbulent motion of glycerol in a container heated from below and cooled from above. The bright lines show regions of rapid temperature variation. The fluid contains many "plumes," especially near the walls. The plumes can be identified as mushroom-shaped objects with heat flowing through the "stalk" and spreading in the "cap." The hot plumes tend to rise with their caps on top; falling, cold plumes are cap-down. All this plume activity is carried along in an overall counterclockwise "wind" caused by convection. Note the thermometer coming down from the top of the cell. Figure adapted from J. Zhang, S. Childress, A. Libchaber, Phys. Fluids 9, 1034 (1997). See detailed discussion in Kadanoff, L. P., Physics Today 54, 34 (August 2001). 5 The temperature of land changes more quickly than the nearby ocean. Thus convective “sea breezes” blow ____ during the day and ____ during the night. A. onshore … onshore B. onshore … offshore C. offshore … onshore D. offshore … offshore 6 The temperature of land changes more quickly than the nearby ocean. Thus convective “sea breezes” blow ____ during the day and ____ during the night. A. onshore … onshore B.onshore … offshore C.offshore … onshore D.offshore … offshore 7 Thermal radiation Any object whose temperature is above zero Kelvin emits energy in the form of electromagnetic radiation Objects both absorb and emit EM radiation continuously, and this phenomenon helps determine the object’s equilibrium temperature 8 The electromagnetic spectrum 9 Thermal radiation We’ll examine this concept some more in chapter 6 10 Why does the Earth cool more quickly on clear nights than it does on cloudy nights? A. -

Hybrid Electric Vehicles: a Review of Existing Configurations and Thermodynamic Cycles
Review Hybrid Electric Vehicles: A Review of Existing Configurations and Thermodynamic Cycles Rogelio León , Christian Montaleza , José Luis Maldonado , Marcos Tostado-Véliz * and Francisco Jurado Department of Electrical Engineering, University of Jaén, EPS Linares, 23700 Jaén, Spain; [email protected] (R.L.); [email protected] (C.M.); [email protected] (J.L.M.); [email protected] (F.J.) * Correspondence: [email protected]; Tel.: +34-953-648580 Abstract: The mobility industry has experienced a fast evolution towards electric-based transport in recent years. Recently, hybrid electric vehicles, which combine electric and conventional combustion systems, have become the most popular alternative by far. This is due to longer autonomy and more extended refueling networks in comparison with the recharging points system, which is still quite limited in some countries. This paper aims to conduct a literature review on thermodynamic models of heat engines used in hybrid electric vehicles and their respective configurations for series, parallel and mixed powertrain. It will discuss the most important models of thermal energy in combustion engines such as the Otto, Atkinson and Miller cycles which are widely used in commercial hybrid electric vehicle models. In short, this work aims at serving as an illustrative but descriptive document, which may be valuable for multiple research and academic purposes. Keywords: hybrid electric vehicle; ignition engines; thermodynamic models; autonomy; hybrid configuration series-parallel-mixed; hybridization; micro-hybrid; mild-hybrid; full-hybrid Citation: León, R.; Montaleza, C.; Maldonado, J.L.; Tostado-Véliz, M.; Jurado, F. Hybrid Electric Vehicles: A Review of Existing Configurations 1. Introduction and Thermodynamic Cycles. -

Development of Miller Cycle Gas Engine for Cogeneration
4. 9 & FR0105396 DEVELOPMENT OF MILLER CYCLE GAS ENGINE FOR COGENERATION DEVELOPPEMENT D UN MOTEUR A GAZ A CYCLE DE MILLER DESTINE A LA COGENERATION N. Tsukida , A.Sakakura, Y.Murata, and K. Okamoto Tokyo Gas CO.,LTD , Japan T.Abe and T. Takemoto Yanmar Diesel Engine CO.,LTD , Japan ABSTRACT We have developed a 300 kW gas engine cogeneration system for practical use that uses natural gas.Using a gas engine operated under conditions with an excess air ratio X =1 that is able to use a three way catalyst to purify the exhaust gases,we were able to achieve high efficiency through the application of the Miller Cycle, as well as a low NOx output. In terms of product specifications, we were able to achieve an electrical efficiency of 34.2% and a heat recovery efficiency of 49.3%, making an overall efficiency of 83.5% as a cogeneration system. RESUME Nous avons developpe un systeme de cogeneration de 300 kW avec moteur au gaz natural et tout a fait utilisable dans la pratique. Avec un moteur a gaz fonctionnant dans les conditions d ’un rapport d ’exces d ’air de X = 1 et capable d ’utiliser un catalyseur trois voies pour purifier les gaz d ’echappement, nous avons ete en mesure d ’obtenir un rendement eleve en appliquant le Cycle de Miller, et d ’obtenir egalement une faible emission d ’oxydes d ’azote. En termes de performances techniques du produit, nous avons obtenu un rendement electrique de 34,2 % et un rendement de recuperation thermique de 49,3 %, ce qui donne un rendement total de 83,5 % pour le systeme de cogeneration. -

Fuel Cells Versus Heat Engines: a Perspective of Thermodynamic and Production
Fuel Cells Versus Heat Engines: A Perspective of Thermodynamic and Production Efficiencies Introduction: Fuel Cells are being developed as a powering method which may be able to provide clean and efficient energy conversion from chemicals to work. An analysis of their real efficiencies and productivity vis. a vis. combustion engines is made in this report. The most common mode of transportation currently used is gasoline or diesel engine powered automobiles. These engines are broadly described as internal combustion engines, in that they develop mechanical work by the burning of fossil fuel derivatives and harnessing the resultant energy by allowing the hot combustion product gases to expand against a cylinder. This arrangement allows for the fuel heat release and the expansion work to be performed in the same location. This is in contrast to external combustion engines, in which the fuel heat release is performed separately from the gas expansion that allows for mechanical work generation (an example of such an engine is steam power, where fuel is used to heat a boiler, and the steam then drives a piston). The internal combustion engine has proven to be an affordable and effective means of generating mechanical work from a fuel. However, because the majority of these engines are powered by a hydrocarbon fossil fuel, there has been recent concern both about the continued availability of fossil fuels and the environmental effects caused by the combustion of these fuels. There has been much recent publicity regarding an alternate means of generating work; the hydrogen fuel cell. These fuel cells produce electric potential work through the electrochemical reaction of hydrogen and oxygen, with the reaction product being water. -

Recording and Evaluating the Pv Diagram with CASSY
LD Heat Physics Thermodynamic cycle Leaflets P2.6.2.4 Hot-air engine: quantitative experiments The hot-air engine as a heat engine: Recording and evaluating the pV diagram with CASSY Objects of the experiment Recording the pV diagram for different heating voltages. Determining the mechanical work per revolution from the enclosed area. Principles The cycle of a heat engine is frequently represented as a closed curve in a pV diagram (p: pressure, V: volume). Here the mechanical work taken from the system is given by the en- closed area: W = − ͛ p ⋅ dV (I) The cycle of the hot-air engine is often described in an idealised form as a Stirling cycle (see Fig. 1), i.e., a succession of isochoric heating (a), isothermal expansion (b), isochoric cooling (c) and isothermal compression (d). This description, however, is a rough approximation because the working piston moves sinusoidally and therefore an isochoric change of state cannot be expected. In this experiment, the pV diagram is recorded with the computer-assisted data acquisition system CASSY for comparison with the real behaviour of the hot-air engine. A pressure sensor measures the pressure p in the cylinder and a displacement sensor measures the position s of the working piston, from which the volume V is calculated. The measured values are immediately displayed on the monitor in a pV diagram. Fig. 1 pV diagram of the Stirling cycle 0210-Wei 1 P2.6.2.4 LD Physics Leaflets Setup Apparatus The experimental setup is illustrated in Fig. 2. 1 hot-air engine . 388 182 1 U-core with yoke . -

RECIPROCATING ENGINES Franck Nicolleau
RECIPROCATING ENGINES Franck Nicolleau To cite this version: Franck Nicolleau. RECIPROCATING ENGINES. Master. RECIPROCATING ENGINES, Sheffield, United Kingdom. 2010, pp.189. cel-01548212 HAL Id: cel-01548212 https://hal.archives-ouvertes.fr/cel-01548212 Submitted on 27 Jun 2017 HAL is a multi-disciplinary open access L’archive ouverte pluridisciplinaire HAL, est archive for the deposit and dissemination of sci- destinée au dépôt et à la diffusion de documents entific research documents, whether they are pub- scientifiques de niveau recherche, publiés ou non, lished or not. The documents may come from émanant des établissements d’enseignement et de teaching and research institutions in France or recherche français ou étrangers, des laboratoires abroad, or from public or private research centers. publics ou privés. Distributed under a Creative Commons Attribution - NonCommercial| 4.0 International License Mechanical Engineering - 14 May 2010 -1- UNIVERSITY OF SHEFFIELD Department of Mechanical Engineering Mappin street, Sheffield, S1 3JD, England RECIPROCATING ENGINES Autumn Semester 2010 MEC403 - MEng, semester 7 - MEC6403 - MSc(Res) Dr. F. C. G. A. Nicolleau MD54 Telephone: +44 (0)114 22 27700. Direct Line: +44 (0)114 22 27867 Fax: +44 (0)114 22 27890 email: F.Nicolleau@sheffield.ac.uk http://www.shef.ac.uk/mecheng/mecheng cms/staff/fcgan/ MEng 4th year Course Tutor : Pr N. Qin European and Year Abroad Tutor : C. Pinna MSc(Res) and MPhil Course Director : F. C. G. A. Nicolleau c 2010 F C G A Nicolleau, The University of Sheffield -2- Combustion engines Table of content -3- Table of content Table of content 3 Nomenclature 9 Introduction 13 Acknowledgement 16 I - Introduction and Fundamentals of combustion 17 1 Introduction to combustion engines 19 1.1 Pistonengines.................................. -

Advanced Power Cycles with Mixtures As the Working Fluid
Advanced Power Cycles with Mixtures as the Working Fluid Maria Jonsson Doctoral Thesis Department of Chemical Engineering and Technology, Energy Processes Royal Institute of Technology Stockholm, Sweden, 2003 Advanced Power Cycles with Mixtures as the Working Fluid Maria Jonsson Doctoral Thesis Department of Chemical Engineering and Technology, Energy Processes Royal Institute of Technology Stockholm, Sweden, 2003 TRITA-KET R173 ISSN 1104-3466 ISRN KTH/KET/R--173--SE ISBN 91-7283-443-9 Contact information: Royal Institute of Technology Department of Chemical Engineering and Technology, Division of Energy Processes SE-100 44 Stockholm Sweden Copyright © Maria Jonsson, 2003 All rights reserved Printed in Sweden Universitetsservice US AB Stockholm, 2003 Advanced Power Cycles with Mixtures as the Working Fluid Maria Jonsson Department of Chemical Engineering and Technology, Energy Processes Royal Institute of Technology, Stockholm, Sweden Abstract The world demand for electrical power increases continuously, requiring efficient and low-cost methods for power generation. This thesis investigates two advanced power cycles with mixtures as the working fluid: the Kalina cycle, alternatively called the ammonia-water cycle, and the evaporative gas turbine cycle. These cycles have the potential of improved performance regarding electrical efficiency, specific power output, specific investment cost and cost of electricity compared with the conventional technology, since the mixture working fluids enable efficient energy recovery. This thesis shows that the ammonia-water cycle has a better thermodynamic performance than the steam Rankine cycle as a bottoming process for natural gas- fired gas and gas-diesel engines, since the majority of the ammonia-water cycle configurations investigated generated more power than steam cycles. -
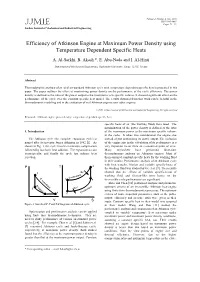
Efficiency of Atkinson Engine at Maximum Power Density Using Temperature Dependent Specific Heats
Volume 2, Number 2, Jun. 2008 ISSN 1995-6665 JJMIE Pages 71 - 75 Jordan Journal of Mechanical and Industrial Engineering Efficiency of Atkinson Engine at Maximum Power Density using Temperature Dependent Specific Heats A. Al-Sarkhi, B. Akash *, E. Abu-Nada and I. Al-Hinti Department of Mechanical Engineering, Hashemite University, Zarqa, 13115, Jordan Abstract Thermodynamic analysis of an ideal air-standard Atkinson cycle with temperature dependant specific heat is presented in this paper. The paper outlines the effect of maximizing power density on the performance of the cycle efficiency. The power density is defined as the ratio of the power output to the maximum cycle specific volume. It showed significant effect on the performance of the cycle over the constant specific heat model. The results obtained from this work can be helpful in the thermodynamic modeling and in the evaluation of real Atkinson engines over other engines. © 2008 Jordan Journal of Mechanical and Industrial Engineering. All rights reserved Keywords: Atkinson engine; power density; temperature dependant specific heat; specific heats of air (the working fluid) were used. The maximization of the power density is defined as the ratio 1. Introduction of the maximum power to the maximum specific volume in the cycle. It takes into consideration the engine size The Atkinson cycle (the complete expansion cycle) is instead of just maximizing its power output. The inclusion named after its inventor James Atkinson in 1882 [1]. As of the engine size in the calculation of its performance is a shown in Fig. 1, the cycle involves isentropic compression very important factor from an economical point of view.