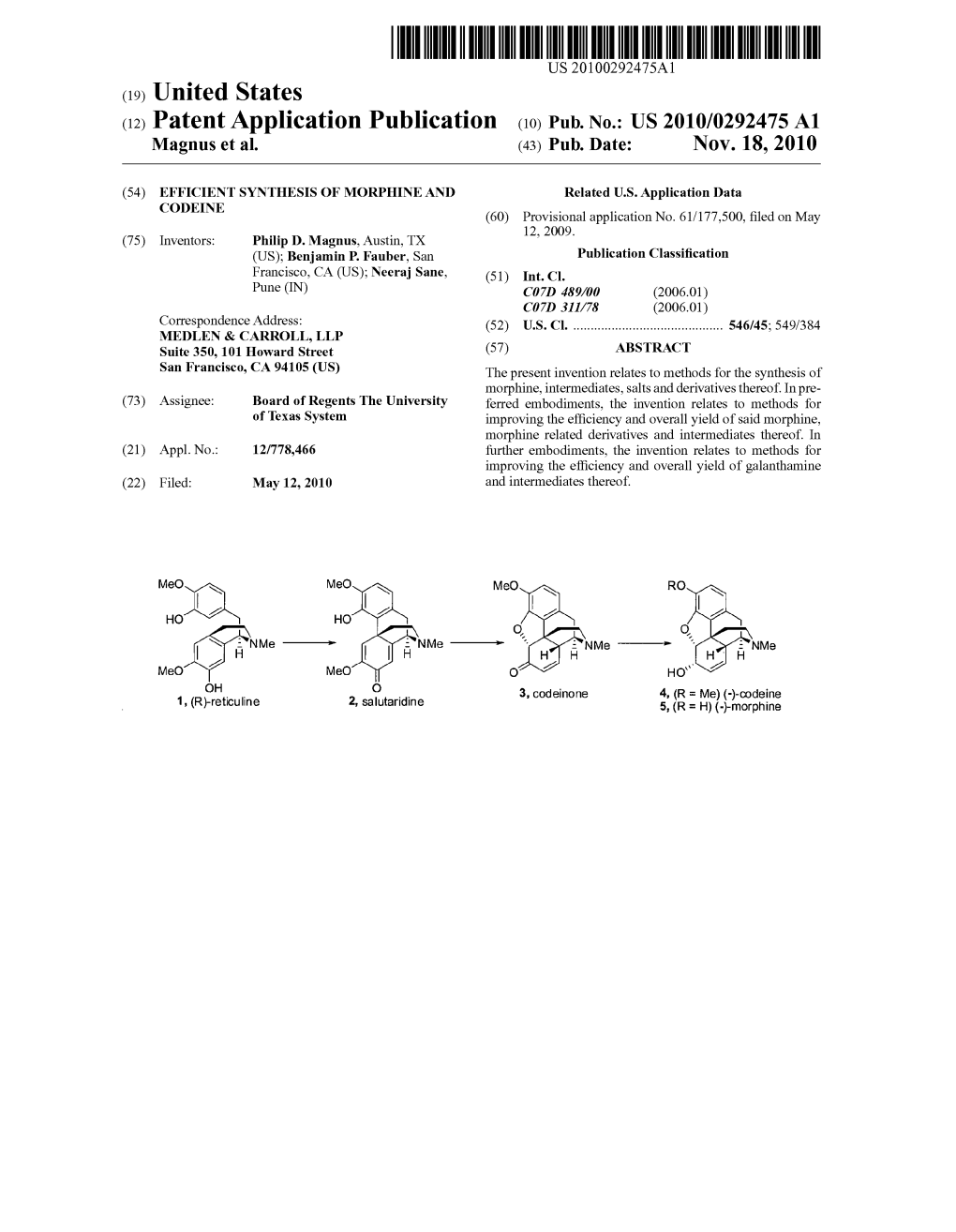
(12) Patent Application Publication (10) Pub. No.: US 2010/0292475A1 Magnus Et Al
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Alternative Formats If You Require This Document in an Alternative Format, Please Contact: [email protected]
University of Bath PHD The extraction and chemistry of the metabolites of Mimosa tenuiflora and Papaver somniferum Ninan, Aleyamma Award date: 1990 Awarding institution: University of Bath Link to publication Alternative formats If you require this document in an alternative format, please contact: [email protected] General rights Copyright and moral rights for the publications made accessible in the public portal are retained by the authors and/or other copyright owners and it is a condition of accessing publications that users recognise and abide by the legal requirements associated with these rights. • Users may download and print one copy of any publication from the public portal for the purpose of private study or research. • You may not further distribute the material or use it for any profit-making activity or commercial gain • You may freely distribute the URL identifying the publication in the public portal ? Take down policy If you believe that this document breaches copyright please contact us providing details, and we will remove access to the work immediately and investigate your claim. Download date: 23. Sep. 2021 THE EXTRACTION AND CHEMISTRY OF THE METABOLITES OF MIMOSA TENUIFLORA AND PAP AVER SOMNIFERUM. submitted by ALEYAMMA NINAN for the degree of Doctor of Philosophy of the University of Bath 1990 Attention is drawn to the fact that the copyright of this thesis rests with its author. This copy of the thesis has been supplied on condition that anyone who consults it is understood to recognise that its copyright rests with its author and that no quotation from the thesis and no information derived from it may be published without prior consent of the author. -

Morphinone Reductase for the Preparation Of
Europäisches Patentamt *EP000649465B1* (19) European Patent Office Office européen des brevets (11) EP 0 649 465 B1 (12) EUROPEAN PATENT SPECIFICATION (45) Date of publication and mention (51) Int Cl.7: C12N 9/02, C12P 17/18, of the grant of the patent: C12Q 1/32 24.07.2002 Bulletin 2002/30 (86) International application number: (21) Application number: 93913236.1 PCT/GB93/01129 (22) Date of filing: 28.05.1993 (87) International publication number: WO 94/00565 (06.01.1994 Gazette 1994/02) (54) MORPHINONE REDUCTASE FOR THE PREPARATION OF HYDROMORPHONE AND HYDROCODONE MORPHINONE REDUKTASE ZUR HERSTELLUNG VON HYDROMORPHONE UND HYDROCODONE MORPHINONE REDUCTASE DESTINEE A LA PREPARATION D’HYDROMORPHONE ET D’HYDROCODONE (84) Designated Contracting States: (74) Representative: Perry, Robert Edward AT BE CH DE DK ES FR GB IE IT LI NL PT SE GILL JENNINGS & EVERY Broadgate House (30) Priority: 25.06.1992 GB 9213524 7 Eldon Street London EC2M 7LH (GB) (43) Date of publication of application: 26.04.1995 Bulletin 1995/17 (56) References cited: WO-A-90/13634 (73) Proprietor: MACFARLAN SMITH LIMITED Edinburgh EH11 2QA (GB) • THE BIOCHEMICAL JOURNAL vol. 274, no. 3, 15 March 1991, pages 875 - 880 NEIL C. BRUCE ET (72) Inventors: AL. ’Microbial degradation of the morphine • HAILES, Anne, Maria 59 Lower Road alkaloids. Purification and characterization of Kent DA8 1AY (GB) morphine dehydrogenase from Pseudomonas • FRENCH, Christopher, Edward putida M10’ Palmerston North (NZ) • BRUCE, Neil, Charles Cambridge CB2 1ND (GB) Note: Within nine months from the publication of the mention of the grant of the European patent, any person may give notice to the European Patent Office of opposition to the European patent granted. -

Effect of Anticancer Agents on Codeinone-Induced Apoptosis in Human Cancer Cell Lines
ANTICANCER RESEARCH 25: 4037-4042 (2005) Effect of Anticancer Agents on Codeinone-induced Apoptosis in Human Cancer Cell Lines RISA TAKEUCHI1,2, HIROSHI HOSHIJIMA1,2, NORIKO ONUKI1,2, HIROSHI NAGASAKA1, SHAHEAD ALI CHOWDHURY3, MASAMI KAWASE4 and HIROSHI SAKAGAMI2 1Division of Anesthesiology, Department of Comprehensive Medical Sciences, 2Division of Pharmacology, Department of Diagnostic and Therapeutic Sciences, and 3MPL (Meikai Phamaco-Medical Laboratory), Meikai University School of Dentistry, Sakado, Saitama 350-0283; 4Faculty of Phamaceutical Sciences, Josai University, Sakado, Saitama 350-0295, Japan Abstract. The possible apoptosis-inducing activity of Opioids have been given to patients with cancer pain, codeinone, an oxidative metabolite of codeine, without or according to the WHO ladder against pain (4). According with anticancer drugs, was investigated. Codeinone induced to reports of Ventafridda et al., pain occurs in more than internucleosomal DNA fragmentation in human 50% of cancer patients, and the administration of opioids promyelocytic leukemia cells (HL-60), but not in human manifests significantly higher therapeutic effects against squamous cell carcinoma cells (HSC-2). Codeinone dose- cancer pain, compared with non-narcotics (5, 6). Although dependently activated caspase-3 in both of these cells, but to opioids have been used for patients in clinical situations, the a much lesser extent than that attained by actinomycin D. anticancer action of opioids has not been well investigated. This property of codeinone was similar to what we have Both opioids and anticancer drugs have been simultaneously found previously in ·,‚-unsaturated ketones. Codeinone did given for pain in cancer patients. However, there has been not activate caspase-8 or caspase-9 in these cells. -

The Identification of Alkaloid Pathway Genes from Non-Model Plant Species in the Amaryllidaceae
Washington University in St. Louis Washington University Open Scholarship Arts & Sciences Electronic Theses and Dissertations Arts & Sciences Winter 12-15-2015 The deI ntification of Alkaloid Pathway Genes from Non-Model Plant Species in the Amaryllidaceae Matthew .B Kilgore Washington University in St. Louis Follow this and additional works at: https://openscholarship.wustl.edu/art_sci_etds Recommended Citation Kilgore, Matthew B., "The deI ntification of Alkaloid Pathway Genes from Non-Model Plant Species in the Amaryllidaceae" (2015). Arts & Sciences Electronic Theses and Dissertations. 657. https://openscholarship.wustl.edu/art_sci_etds/657 This Dissertation is brought to you for free and open access by the Arts & Sciences at Washington University Open Scholarship. It has been accepted for inclusion in Arts & Sciences Electronic Theses and Dissertations by an authorized administrator of Washington University Open Scholarship. For more information, please contact [email protected]. WASHINGTON UNIVERSITY IN ST. LOUIS Division of Biology and Biomedical Sciences Plant Biology Dissertation Examination Committee: Toni Kutchan, Chair Elizabeth Haswell Jeffrey Henderson Joseph Jez Barbara Kunkel Todd Mockler The Identification of Alkaloid Pathway Genes from Non-Model Plant Species in the Amaryllidaceae by Matthew Benjamin Kilgore A dissertation presented to the Graduate School of Arts & Sciences of Washington University in partial fulfillment of the requirements for the degree of Doctor of Philosophy December 2015 St. Louis, Missouri -

(12) United States Patent (10) Patent No.: US 6,469,170 B1 Chiu Et Al
USOO646917OB1 (12) United States Patent (10) Patent No.: US 6,469,170 B1 Chiu et al. (45) Date of Patent: Oct. 22, 2002 (54) METHOD FOR PREPARING OXYCODONE (58) Field of Search ............................................ 546/45 (75) Inventors: Fang-Ting Chiu, Chesterfield, VA (56) References Cited (US); Young S. Lo, Chester, VA (US) FOREIGN PATENT DOCUMENTS (73) Assignee: Boehringer Ingelheim Chemicals, Inc., Petersburg, VA (US) WO 9902529 * 1/1999 (*) Notice: Subject to any disclaimer, the term of this * cited by examiner patent is extended or adjusted under 35 U.S.C. 154(b) by 0 days. Primary Examiner Alan L. Rotman ASSistant Examiner Binta Robinson (21) Appl. No.: 10/152,140 (74) Attorney, Agent, or Firm-Robert P. Raymond; Mary-Ellen M. Devlin; Alan R. Stempel (22)22) FileFilled: Maway Zl,21, 2002 (57) ABSTRACT Related U.S. Application Data A method for the preparation of oxycodone, and Salts (62) Division of application No. 09/793,024, filed on Feb. 26 thereof, from codeine comprising oxidation of codeine to 2001, now Pat. No. 6.403.798, which is a division of codeinone, formation of an dienolsilyl ether congener of application No. 09/667.997, filed on Sep. 22, 2000, now Pat. codeinone in Strong amine base, oxidation of the dienolsilyl No. 6,262,266, which is a division of application No. ether congener using peracetic acid, and hydrogenation of 09/419,429, filed on Oct. 15, 1999, now Pat. No. 6,177.567. the resulting 14-hydroxycodeinone product. (51) Int. Cl." .............................................. C07D 471/00 (52) U.S. Cl. ......................................................... 546/45 8 Claims, No Drawings US 6,469,170 B1 1 METHOD FOR PREPARING OXYCODONE BACKGROUND OF THE INVENTION 1. -

The Marvels of Biosynthesis: Tracking Nature's Pathways
Pergamon Bioorganic & Medicinal Chemistry, Vol. 4, No. 7, pp 937-964, 1996 Copyright © 1996 Elsevier Science Ltd Printed in Great Britain. All rights reserved PIh S0968-0896(96)00102-2 0968-0896/96 $15.00+0.00 The Marvels of Biosynthesis: Tracking Nature's Pathways Alan R. Battersby University of Cambridge, University Chemical Laboratory, Lensfield Road, Cambridge CB2 1EW, U.K. Introduction and nitric acids, zinc, sulphur, copper sulphate and many more materials. Those days are gone and there How ever did it come about that a substantial part of are pluses and minuses to the change. At any rate, I my research has been aimed at understanding the was able to assemble a good set of equipment to run marvellous chemistry used by living systems to lots of simple experiments which I enjoyed enormously. ,:onstruct the substances they produce? I must admit ".hat I had not in the past thought much about that I believe the next important influence on me came at 9articular 'pathway' but was encouraged to do so by school where I had the great good fortune to be taught Derek Barton, Chairman of the Executive Board of more about chemistry by a superb teacher, Mr Evans. Editors for Tetrahedron Publications. He suggested The seed of my love for chemistry which had been that this article, invited by Professor Chi-Huey Wong, planted earlier by my father's books was strongly fed by should be a personal one giving some background on his teaching. Then I read my first books about organic how my interests evolved. -

Ep001837396a1*
(19) *EP001837396A1* (11) EP 1 837 396 A1 (12) EUROPEAN PATENT APPLICATION (43) Date of publication: (51) Int Cl.: 26.09.2007 Bulletin 2007/39 C12N 9/02 (2006.01) C12N 15/53 (2006.01) C12N 15/82 (2006.01) C12N 5/14 (2006.01) (2006.01) (2006.01) (21) Application number: 06006045.6 C07K 16/16 C12P 21/02 C07D 489/02 (2006.01) A61K 38/44 (2006.01) (22) Date of filing: 23.03.2006 (84) Designated Contracting States: (72) Inventors: AT BE BG CH CY CZ DE DK EE ES FI FR GB GR • Ziegler, Jörg, Dr. HU IE IS IT LI LT LU LV MC NL PL PT RO SE SI 06112 Halle (DE) SK TR • Kutchan, Toni M. Prof. Dr. Designated Extension States: St. Louis, Missouri 63141 (US) AL BA HR MK YU (74) Representative: Grünecker, Kinkeldey, (71) Applicant: Leibniz-Institut für Pflanzenbiochemie Stockmair & Schwanhäusser (IPB) Anwaltssozietät 06120 Halle (Saale) (DE) Maximilianstrasse 58 80538 München (DE) (54) Salutaridine reductase and morphine biosynthesis (57) Plants of the order Ranunculales, especially viously isolated cDNAs coding for enzymes in benzyliso- members of the species Papaver, accumulate a large quinoline biosynthesis and which showed the highest variety of benzylisoquinoline alkaloids with about 2,500 amino acid identity to reductases in menthol biosynthe- structures. But only the opium poppy, Papaver somni- sis. When expressed, the protein encoded by this cDNA ferum, and Papaver setigerum, are able to produce mor- reduced the keto group of salutaridine yielding salutar- phinan alkaloids such as the analgesic morphine or the idinol, an intermediate in morphine biosynthesis. -

Division of Vital Statistics, Mortality Data Table Page 1 of 147
Division of Vital Statistics, Mortality Data Table page 1 of 147 Table I–1. Search terms to identify drugs mentioned in electronic death certificate literal text and associated principal variant, Version 06-30-2015 Spreadsheet version available from: ftp://ftp.cdc.gov/pub/Health_Statistics/NCHS/Publications/NVSR/65_09/Table01.xlsx. Search term Principal variant A PRAZOLAM ALPRAZOLAM ABACAVIR ABACAVIR ABARELIX ABARELIX ABATACEPT ABATACEPT ABCIXIMAB ABCIXIMAB ABILIFY ARIPIPRAZOLE ABIRATERONE ABIRATERONE ABOBOTULINUMTOXINA ABOBOTULINUMTOXINA ABSTRAL FENTANYL ACAMPROSATE ACAMPROSATE ACARBOSE ACARBOSE ACDETAMINOPHEN ACETAMINOPHEN ACEBUTOLOL ACEBUTOLOL ACEPROMAZINE ACEPROMAZINE ACETAMINOP ACETAMINOPHEN ACETAMINOPHEN ACETAMINOPHEN ACETAZOLAMIDE ACETAZOLAMIDE ACETIC ACID ACETIC ACID ACETOHEXAMIDE ACETOHEXAMIDE ACETOHYDROXAMIC ACID ACETOHYDROXAMIC ACID ACETOMINOPHEN ACETAMINOPHEN ACETONE ACETONE ACETOPHENAZINE ACETOPHENAZINE ACETYL MORPHINE HEROIN ACETYLCARBROMAL ACETYLCARBROMAL ACETYLCHOLINE ACETYLCHOLINE ACETYLCYSTEINE ACETYLCYSTEINE ACETYLSALICYCLIC ACID ACETYLSALICYLIC ACID ACETYLSALICYLACID ACETYLSALICYLIC ACID ACETYLSALICYLIC ACID ACETYLSALICYLIC ACID ACETYSALICYLIC ACID ACETYLSALICYLIC ACID ACITRETIN ACITRETIN ACRIFLAVINE ACRIFLAVINE ACRIVASTINE ACRIVASTINE ACTIQ FENTANYL ACYCLOVIR ACYCLOVIR ADALIMUMAB ADALIMUMAB ADANON METHADONE ADAPALENE ADAPALENE ADDERALL AMPHETAMINE DEXTROAMPHETAMINE ADEFOVIR ADEFOVIR AFATINIB AFATINIB U.S. Department of Health and Human Services · Centers for Disease Control and Prevention · National Center for Health -

Comparisons of in Vivo and in Vitro Opioid Effects of Newly Synthesized
molecules Article Comparisons of In Vivo and In Vitro Opioid Effects of Newly Synthesized 14-Methoxycodeine-6-O-sulfate and Codeine-6-O-sulfate Ferenc Zádor 1,2, Amir Mohammadzadeh 1, Mihály Balogh 1, Zoltán S. Zádori 1 , Kornél Király 1, Szilvia Barsi 1 , Anna Rita Galambos 1, Szilvia B. László 1, Barbara Hutka 1, András Váradi 3, Sándor Hosztafi 3,Pál Riba 1,Sándor Benyhe 2, Susanna Fürst 1 and Mahmoud Al-Khrasani 1,* 1 Department of Pharmacology and Pharmacotherapy, Faculty of Medicine, Semmelweis University, Nagyvárad tér 4, P.O. Box 370, H-1445 Budapest, Hungary; [email protected] (F.Z.); [email protected] (A.M.); [email protected] (M.B.); [email protected] (Z.S.Z.); [email protected] (K.K.); [email protected] (S.B.); [email protected] (A.R.G.); [email protected] (S.B.L.); [email protected] (B.H.); [email protected] (P.R.); [email protected] (S.F.) 2 Institute of Biochemistry, Biological Research Center of the Hungarian Academy of Sciences, Temesvári krt. 62., H-6726 Szeged, Hungary; [email protected] 3 Department of Pharmaceutical Chemistry, Semmelweis University, H˝ogyesEndre u., 9. H-1092 Budapest, Hungary; [email protected] (A.V.); hosztafi[email protected] (S.H.) * Correspondence: [email protected]; Tel.: +36-1-2104-416 Received: 15 February 2020; Accepted: 15 March 2020; Published: 17 March 2020 Abstract: The present work represents the in vitro (potency, affinity, efficacy) and in vivo (antinociception, constipation) opioid pharmacology of the novel compound 14-methoxycodeine-6- O-sulfate (14-OMeC6SU), compared to the reference compounds codeine-6-O-sulfate (C6SU), codeine and morphine. -

14-O-Methylmorphine a Novel Selective Mu-Opioid Receptor
European Journal of Pharmacology xxx (xxxx) xxx–xxx Contents lists available at ScienceDirect European Journal of Pharmacology journal homepage: www.elsevier.com/locate/ejphar Full length article 14-O-Methylmorphine: A Novel Selective Mu-Opioid Receptor Agonist with High Efficacy and Affinity Ferenc Zádorb,1, Mihály Balogha,1, András Váradic, Zoltán S. Zádoria, Kornél Királya, Edina Szűcsb, Bence Vargaa, Bernadette Lázára, Sándor Hosztafic, Pál Ribaa, Sándor Benyheb, ⁎ Susanna Fürsta, Mahmoud Al-Khrasania, a Department of Pharmacology and Pharmacotherapy, Faculty of Medicine, Semmelweis University, Nagyvárad tér 4, P.O. Box 370, H-1445 Budapest, Hungary b Institute of Biochemistry, Biological Research Centre of the Hungarian Academy of Sciences, Temesvári krt. 62., H- 6726 Szeged, Hungary c Department of Pharmaceutical Chemistry, Semmelweis University, Hőgyes Endre u., 9. H-1092 Budapest, Hungary ARTICLE INFO ABSTRACT Keywords: 14-O-methyl (14-O-Me) group in morphine-6-O-sulfate (M6SU) or oxymorphone has been reported to be es- 14-O-methylmorphine sential for enhanced affinity, potency and antinociceptive effect of these opioids. Herein we report on the Morphine pharmacological properties (potency, affinity and efficacy) of the new compound, 14-O-methylmorphine (14-O- Receptor binding MeM) in in vitro. Additionally, we also investigated the antinociceptive effect of the novel compound, as well as Intrinsic efficacy its inhibitory action on gastrointestinal transit in in vivo. The potency and efficacy of test compound were Antinociception measured by [35S]GTPγS binding, isolated mouse vas deferens (MVD) and rat vas deferens (RVD) assays. The Gastrointestinal transit affinity of 14-O-MeM for opioid receptors was assessed by radioligand binding and MVD assays. -

Codeine Epoxide, a New Metabolite of Codeine, and Epoxides of Morphine and Heroin on the Electrically Stimulated Guinea Pig Ileum Were Studied
EFFECTS OF EPOXIDES OF HEROIN, MORPHINE AND CODEINE ON THE ELECTRICALLY STIMULATED GUINEA PIG ILEUM Issei TAKAYANAGI, Naoki MIYATA*, Kiyoko UBA*, Keizo WATANABE* and Masaaki HIROBE* Department of Chemical Pharmacology, Toho University School of Pharmaceutical Sciences , Miyama, Funabashi, Chiba 274, Japan and *Department of Bioorganic and Medicinal chemistry , Faculty of Pharmaceutical Sciences, University of Tokyo, Bunkyo-ku , Tokyo 113, Japan Accepted March 6, 1981 Abstract-Modes of action of codeine epoxide, a new metabolite of codeine, and epoxides of morphine and heroin on the electrically stimulated guinea pig ileum were studied. The opiates and their epoxides inhibited the twitch response of the ileum to electrical stimulation. The inhibitory actions of the test drugs were antagonized by naloxone. The activities of epoxides were not strikingly different from those of the parent opiates. Dependence liabilities of the epoxides were also tested on the guinea pig ileum which was treated with a high concentration of the test drugs. Epoxydation of 7,8-double bond in the parent opiates showed a trend toward a decrease in dependence liability. Codeine-7,8-oxide (codeine epoxide) has, suspended in a 20 ml organ bath filled with recently, been identified as a new metabolite Locke Ringer solution kept at 32°C and of codeine in the rat (1). Derivatives of gassed with a mixture of 95% oxygen and 5% 7,8-oxides of morphine and heroin are carbon dioxide. The twitch responses of the assumed to be metabolites of morphine and ileum to electrical stimulation were recorded heroin. As opiate receptors in the chol isometrically under an initial tension of 1.0 g. -

Discovery of Novel Opioid Medications
National Institute on Drug Abuse RESEARCH MONOGRAPH SERIES Discovery of Novel Opioid Medications 147 U.S. Department of Health and Human Services • Public Health Service • National Institutes of Health Discovery of Novel Opioid Medications Editors: Rao S. Rapaka, Ph.D. Heinz Sorer, Ph.D. NIDA Research Monograph 147 1995 U.S. DEPARTMENT OF HEALTH AND HUMAN SERVICES Public Health Service National Institutes of Health National Institute on Drug Abuse 5600 Fishers Lane Rockville, MD 20857 ACKNOWLEDGEMENT This monograph is based on the papers from a technical review on “Discovery of Novel Opioid Medications” held on July 28-29, 1993. The review meeting was sponsored by the National Institute on Drug Abuse. COPYRIGHT STATUS The National Institute on Drug Abuse has obtained permission from the copyright holders to reproduce certain previously published material as noted in the text. Further reproduction of this copyrighted material is permitted only as part of a reprinting of the entire publication or chapter. For any other use, the copyright holder’s permission is required. All other material in this volume except quoted passages from copyrighted sources is in the public domain and may be used or reproduced without permission from the Institute or the authors. Citation of the source is appreciated. Opinions expressed in this volume are those of the authors and do not necessarily reflect the opinions or official policy of the National Institute on Drug Abuse or any other part of the U.S. Department of Health and Human Services. The U.S. Government does not endorse or favor any specific commercial product or company.