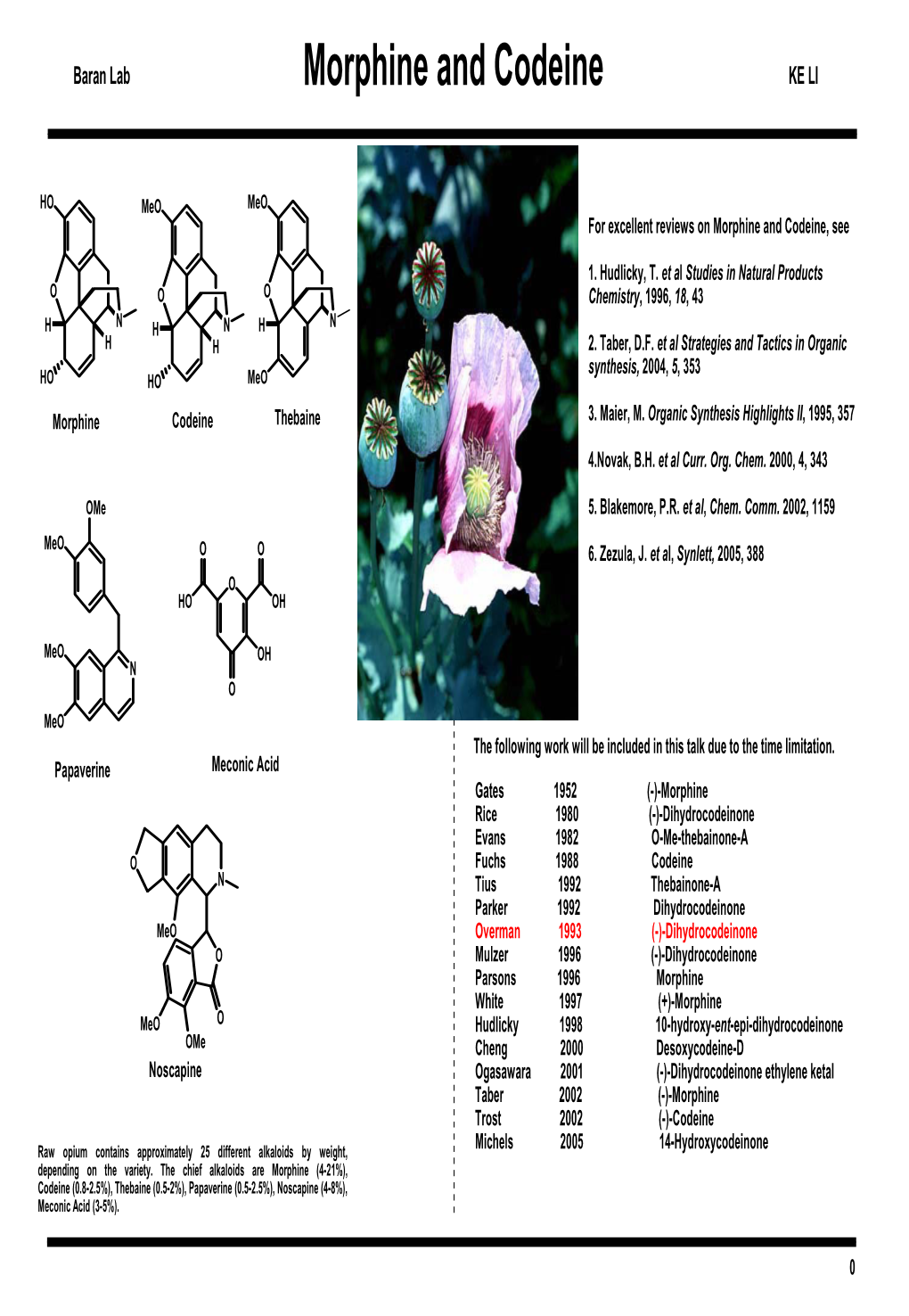
Morphine and Codeine, See
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Alternative Formats If You Require This Document in an Alternative Format, Please Contact: [email protected]
University of Bath PHD The extraction and chemistry of the metabolites of Mimosa tenuiflora and Papaver somniferum Ninan, Aleyamma Award date: 1990 Awarding institution: University of Bath Link to publication Alternative formats If you require this document in an alternative format, please contact: [email protected] General rights Copyright and moral rights for the publications made accessible in the public portal are retained by the authors and/or other copyright owners and it is a condition of accessing publications that users recognise and abide by the legal requirements associated with these rights. • Users may download and print one copy of any publication from the public portal for the purpose of private study or research. • You may not further distribute the material or use it for any profit-making activity or commercial gain • You may freely distribute the URL identifying the publication in the public portal ? Take down policy If you believe that this document breaches copyright please contact us providing details, and we will remove access to the work immediately and investigate your claim. Download date: 23. Sep. 2021 THE EXTRACTION AND CHEMISTRY OF THE METABOLITES OF MIMOSA TENUIFLORA AND PAP AVER SOMNIFERUM. submitted by ALEYAMMA NINAN for the degree of Doctor of Philosophy of the University of Bath 1990 Attention is drawn to the fact that the copyright of this thesis rests with its author. This copy of the thesis has been supplied on condition that anyone who consults it is understood to recognise that its copyright rests with its author and that no quotation from the thesis and no information derived from it may be published without prior consent of the author. -

“Biosynthesis of Morphine in Mammals”
“Biosynthesis of Morphine in Mammals” D i s s e r t a t i o n zur Erlangung des akademischen Grades Doctor rerum naturalium (Dr. rer. nat.) vorgelegt der Naturwissenschaftlichen Fakultät I Biowissenschaften der Martin-Luther-Universität Halle-Wittenberg von Frau Nadja Grobe geb. am 21.08.1981 in Querfurt Gutachter /in 1. 2. 3. Halle (Saale), Table of Contents I INTRODUCTION ........................................................................................................1 II MATERIAL & METHODS ........................................................................................ 10 1 Animal Tissue ....................................................................................................... 10 2 Chemicals and Enzymes ....................................................................................... 10 3 Bacteria and Vectors ............................................................................................ 10 4 Instruments ........................................................................................................... 11 5 Synthesis ................................................................................................................ 12 5.1 Preparation of DOPAL from Epinephrine (according to DUNCAN 1975) ................. 12 5.2 Synthesis of (R)-Norlaudanosoline*HBr ................................................................. 12 5.3 Synthesis of [7D]-Salutaridinol and [7D]-epi-Salutaridinol ..................................... 13 6 Application Experiments ..................................................................................... -

Seized Drugs Technical Manual, 12-08-2020
Seized Drugs Technical Manual Approval Date: 12/08/2020 Document Number: 4561 Approved By: David Gouldthorpe, Kim Murga, Cassandra Robertson Revision Number: 17 Date Published: 12/08/2020 Las Vegas Metropolitan Police Department Forensic Laboratory 5605 W. Badura Ave. Ste. 120B Las Vegas, NV 89118 Seized Drugs Technical Manual Uncontrolled Copy if not located in Qualtrax Page 1 of 166 Seized Drugs Technical Manual Approval Date: 12/08/2020 Document Number: 4561 Approved By: David Gouldthorpe, Kim Murga, Cassandra Robertson Revision Number: 17 Date Published: 12/08/2020 Table of Contents Chapter Title Introduction ANALYTICAL TECHNIQUES 1.1 Color Tests 1.2 Chromatography 1.3 Mass Spectrometry (MS) 1.4 Infrared Spectroscopy (IR) 1.5 X-Ray Fluorescence (XRF) 1.6 Raman Spectroscopy QUALITY CONTROL 2.1 Reference Materials and Supplies 2.2 Reference Material Inventory Audit 2.3 Color Test Reagent Quality Control 2.4 Quality Control Plan SEIZED DRUG ANALYSIS 3.1 Seized Drugs Analysis Quality Control 3.2 Sampling 3.3 Identification Criteria 3.4 Evidence Discrepancies and Preliminary Field Test Errors 3.5 Cannabis Analysis 3.6 Opium Analysis 3.7 Analysis of Fentanyl and Fentanyl Related Substances CLANDESTINE LABORATORIES 4.1 Clandestine Laboratory Response 4.2 Clandestine Laboratory Analysis REPORTING AND TECHNICAL REVIEW 5.1 Reporting 5.2 Technical Review 6 Retraining 7 Recipes and Derivatizing Agents PROCEDURES 8.1 Logging Reference Materials into LIMS 8.2 Verifying Reference Materials Uncontrolled Copy if not located in Qualtrax Page 2 of 166 -

Tommann Tuntimahon At
TOMMANNUS009909137B2TUNTIMAHON AT THE (12 ) United States Patent ( 10 ) Patent No. : US 9 , 909 , 137 B2 Fist (45 ) Date of Patent: *Mar . 6 , 2018 ( 54 ) PAPAVER SOMNIFERUM STRAIN WITH OTHER PUBLICATIONS HIGH CONCENTRATION OF THEBAINE Allen , R . S . , et al . , “Metabolic engineering of morphinan alkaloids (71 ) Applicant: Tasmanian Alkaloids Pty . Ltd ., by over -expression and RNAi suppression of salutaridinol 7 - 0 Westbury , Tasmania ( AU ) acetyltransferase in opium poppy ” Plant Biotechnology Journal; Jan . 2008 , vol. 6 , pp . 22 - 30 . (72 ) Inventor : Anthony J . Fist , Norwood (AU ) Patra , N . K . and Chavhan S . P ., “ Morphophysiology and geneticsof induced mutants expressed in the M1 generation in opium poppies , ” ( 73 ) Assignee : Tasmanian Alkaloids Pty . Ltd . , The Journal of Heredity ( 1990 ) ; 81( 5 ) pp . 347 - 350 . Crane , F . A . and Fairburn J . W . , “ Alkaloids in the germinating Westbury , Tasmania (AU ) seedling of poppy " , Transactions of the Illinois State Academy of Science . ( 1970 ) vol. 63 , No . 1 , 11 pp . 86 - 92 . ( * ) Notice : Subject to any disclaimer, the term of this Kutchan , T . M . and Dittrich H ., “ Characterization and mechanism of patent is extended or adjusted under 35 the berberine bridge enzyme, a covalently flavivated oxidase of U . S . C . 154 (b ) by 69 days . benzophenanthridine alkaloid biosynthesis in plants, ” ( 1995 ) vol. 270 , No. 41 Issue Oct. 13 ; pp . 24475 - 24481 . This patent is subject to a terminal dis Facchini, P . J . et al ., “ Molecular characterization of berberine bridge claimer. enzyme genes from opium poppy, ” ( 1996 ) Plant Physiol. 112: pp . 1669 - 1677 . De- Eknamkul W . and Zenk M . H ., “ Enzymatic formulation of (21 ) Appl . No. : 14 /816 ,639 ( R ) - reticuline from 1 , 2 - dehydroreticuline in the opium poppy plant, ” ( 1990 ) Tetradedron Lett 31 ( 34 ) , 4855 - 8 . -

1. Introduction
Introduction 1. Introduction 1.1. Alkaloids The term alkaloid is derived from Arabic word al-qali, the plant from which “soda” was first obtained (Kutchan, 1995). Alkaloids are a group of naturally occurring low-molecular weight nitrogenous compounds found in about 20% of plant species. The majority of alkaloids in plants are derived from the amino acids tyrosine, tryptophan and phenylalanine. They are often basic and contain nitrogen in a heterocyclic ring. The classification of alkaloids is based on their carbon-nitrogen skeletons; common alkaloid ring structures include the pyridines, pyrroles, indoles, pyrrolidines, isoquinolines and piperidines (Petterson et al., 1991; Bennett et al., 1994). In nature, plant alkaloids are mainly involved in plant defense against herbivores and pathogens. Many of these compounds have biological activity which makes them suitable for use as stimulants (nicotine, caffeine), pharmaceuticals (vinblastine), narcotics (cocaine, morphine) and poisons (tubocurarine). The discovery of morphine by the German pharmacist Friedrich W. Sertürner in 1806 began the field of plant alkaloid biochemistry. However, the structure of morphine was not determined until 1952 due to its stereochemical complexity. Major technical advances occurred in this field allowing for the elucidation of selected alkaloid biosynthetic pathways. Among these were the introduction of radiolabeled precursors in the 1950s and the establishment in the 1970s of plant cell suspension cultures as an abundant source of enzymes that could be isolated, purified and characterized. Finally, the introduction of molecular techniques has made possible the isolation of genes involved in alkaloid secondary pathways (Croteau et al., 2000; Facchini, 2001). 1.1.1. Benzylisoquinoline alkaloids Isoquinoline alkaloids represent a large and varied group of physiologically active natural products. -

Morphinone Reductase for the Preparation Of
Europäisches Patentamt *EP000649465B1* (19) European Patent Office Office européen des brevets (11) EP 0 649 465 B1 (12) EUROPEAN PATENT SPECIFICATION (45) Date of publication and mention (51) Int Cl.7: C12N 9/02, C12P 17/18, of the grant of the patent: C12Q 1/32 24.07.2002 Bulletin 2002/30 (86) International application number: (21) Application number: 93913236.1 PCT/GB93/01129 (22) Date of filing: 28.05.1993 (87) International publication number: WO 94/00565 (06.01.1994 Gazette 1994/02) (54) MORPHINONE REDUCTASE FOR THE PREPARATION OF HYDROMORPHONE AND HYDROCODONE MORPHINONE REDUKTASE ZUR HERSTELLUNG VON HYDROMORPHONE UND HYDROCODONE MORPHINONE REDUCTASE DESTINEE A LA PREPARATION D’HYDROMORPHONE ET D’HYDROCODONE (84) Designated Contracting States: (74) Representative: Perry, Robert Edward AT BE CH DE DK ES FR GB IE IT LI NL PT SE GILL JENNINGS & EVERY Broadgate House (30) Priority: 25.06.1992 GB 9213524 7 Eldon Street London EC2M 7LH (GB) (43) Date of publication of application: 26.04.1995 Bulletin 1995/17 (56) References cited: WO-A-90/13634 (73) Proprietor: MACFARLAN SMITH LIMITED Edinburgh EH11 2QA (GB) • THE BIOCHEMICAL JOURNAL vol. 274, no. 3, 15 March 1991, pages 875 - 880 NEIL C. BRUCE ET (72) Inventors: AL. ’Microbial degradation of the morphine • HAILES, Anne, Maria 59 Lower Road alkaloids. Purification and characterization of Kent DA8 1AY (GB) morphine dehydrogenase from Pseudomonas • FRENCH, Christopher, Edward putida M10’ Palmerston North (NZ) • BRUCE, Neil, Charles Cambridge CB2 1ND (GB) Note: Within nine months from the publication of the mention of the grant of the European patent, any person may give notice to the European Patent Office of opposition to the European patent granted. -

Chemistry Exam - 2015
Scientific Officer Chemistry Exam - 2015 (Final Answer Key) Chemistry Q.No: 1 The major detoxification reaction involved in Phase - I are all except. A Oxidation B Hydrolysis C Acetylation D Hydroxylation Q.No: 2 Which of the endogenous substance is detoxified through glucuronidation reaction ? A Aspirin B Methanol C Bilirubin D Phenyl Acetate Q.No: 3 Which of the following is not a conjugating agent in drug metabolisim? A Active acetate B Active sulfate C Active glucuronate D Active Bicarbonate Q.No: 4 Which of the following statements is not true about receptors ? A Most receptors are proteins situated in the cell membrane B Receptors contain a cleft- known as binding site C Receptors catalyze reactions on chemical messengers D Receptors bind to chemical messengers e.g. neurotransmitters etc Q.No: 5 The mechanism behind pesticide poisoning is A Irreversible binding with Acetyl choline esterase B Reversible binding with Acetyl choline esterase C Irreversible binding with nicotinic receptors D Reversible binding with nicotinic receptors Q.No: 6 Which one of the following have binding affinity but no intrinsic activity? A Agonist B Antagonist C Partial Agonist D Inverse Agonist Q.No: 7 Which of the following is not a requirement for a drug to act as an agonist ? A Functional group B Metabolic stability C Pharmacophore D Size Q.No: 8 Polycyclic aromatic hydrocarbons (PAH) in cigarette smoke may cause - A Inhibition of cytochrome P-450 and delayed metabolism B Inhibition of cytochrome P-450 and enhanced metabolism C Induction of cytochrome -

By HENRYDEANE, F.L.S., and HENRYB
View Article Online / Journal Homepage / Table of Contents for this issue 34 DEASE AND BRADY ON MICROSCOPICAL VIIP.-On ikIicroscopicu2 Research in relation to Pharmacy. By HENRYDEANE, F.L.S., and HENRYB. BRADY,F.L.S. [Read at the Bath Meeting of the British Pharmaceutical Conference, Sept., 1864.1 WE have chosen for the particular subject of the present commu- nication the various preparations of opium. Whether regarded in respect to their importance in the practice of medicine, their variability in strength and character, or the peculiar conditions in which the active matter exists in the crude drug, no better subject could be found for the purpose in view. Opium, as is well known, is an extremely composite substance, being a pasty mass formed of resinous, gummy, extractive and albuminous matters, containing a larger or srrialler percentage of certain active principles diffused through it. These principles are morphine, narcotine (with its two homologuesj, codeine, narceine, mecoiiine, thellaine, and papaverine, either existing free or in com- bination with meconic, sulphuric, or other acids, the sum of the crystalline constituents, exclusive of inorganic salts, contained in good samples of the drug, being from twenty to thirty per cent. of its entire weight. Any preparation, exactly to represent opium, must contain the whole of' these principles, as indeed the tincture may be said fairly to do. It, has, however, been shown that some of the principles are inert, and others even deleterious in their action, and we have Published on 01 January 1865. Downloaded by University of Pittsburgh 30/10/2014 05:40:05. -

Effect of Anticancer Agents on Codeinone-Induced Apoptosis in Human Cancer Cell Lines
ANTICANCER RESEARCH 25: 4037-4042 (2005) Effect of Anticancer Agents on Codeinone-induced Apoptosis in Human Cancer Cell Lines RISA TAKEUCHI1,2, HIROSHI HOSHIJIMA1,2, NORIKO ONUKI1,2, HIROSHI NAGASAKA1, SHAHEAD ALI CHOWDHURY3, MASAMI KAWASE4 and HIROSHI SAKAGAMI2 1Division of Anesthesiology, Department of Comprehensive Medical Sciences, 2Division of Pharmacology, Department of Diagnostic and Therapeutic Sciences, and 3MPL (Meikai Phamaco-Medical Laboratory), Meikai University School of Dentistry, Sakado, Saitama 350-0283; 4Faculty of Phamaceutical Sciences, Josai University, Sakado, Saitama 350-0295, Japan Abstract. The possible apoptosis-inducing activity of Opioids have been given to patients with cancer pain, codeinone, an oxidative metabolite of codeine, without or according to the WHO ladder against pain (4). According with anticancer drugs, was investigated. Codeinone induced to reports of Ventafridda et al., pain occurs in more than internucleosomal DNA fragmentation in human 50% of cancer patients, and the administration of opioids promyelocytic leukemia cells (HL-60), but not in human manifests significantly higher therapeutic effects against squamous cell carcinoma cells (HSC-2). Codeinone dose- cancer pain, compared with non-narcotics (5, 6). Although dependently activated caspase-3 in both of these cells, but to opioids have been used for patients in clinical situations, the a much lesser extent than that attained by actinomycin D. anticancer action of opioids has not been well investigated. This property of codeinone was similar to what we have Both opioids and anticancer drugs have been simultaneously found previously in ·,‚-unsaturated ketones. Codeinone did given for pain in cancer patients. However, there has been not activate caspase-8 or caspase-9 in these cells. -

Structure of a Berberine Bridge Enzyme-Like Enzyme with an Active Site Specific to the Plant Family Brassicaceae
Structure of a Berberine Bridge Enzyme-Like Enzyme with an Active Site Specific to the Plant Family Brassicaceae Daniel, Bastian; Wallner, Silvia; Steiner, Barbara; Oberdorfer, Gustav; Kumar, Prashant; van der Graaff, Eric; Roitsch, Thomas; Sensen, Christoph W; Gruber, Karl; Macheroux, Peter Published in: PLOS ONE DOI: 10.1371/journal.pone.0156892 Publication date: 2016 Document version Publisher's PDF, also known as Version of record Citation for published version (APA): Daniel, B., Wallner, S., Steiner, B., Oberdorfer, G., Kumar, P., van der Graaff, E., ... Macheroux, P. (2016). Structure of a Berberine Bridge Enzyme-Like Enzyme with an Active Site Specific to the Plant Family Brassicaceae. PLOS ONE, 11(6), e0156892. https://doi.org/10.1371/journal.pone.0156892 Download date: 08. Apr. 2020 RESEARCH ARTICLE Structure of a Berberine Bridge Enzyme-Like Enzyme with an Active Site Specific to the Plant Family Brassicaceae Bastian Daniel1, Silvia Wallner1, Barbara Steiner1, Gustav Oberdorfer2, Prashant Kumar2, Eric van der Graaff3, Thomas Roitsch3,4, Christoph W. Sensen5, Karl Gruber2, Peter Macheroux1* 1 Institute of Biochemistry, Graz University of Technology, Graz, Austria, 2 Institute of Molecular Biosciences, University of Graz, Graz, Austria, 3 Department of Plant and Environmental Sciences, a11111 University of Copenhagen, Copenhagen, Denmark, 4 Global Change Research Centre, Czech Globe AS CR, v.v.i., Drásov 470, Cz-664 24 Drásov, Czech Republic, 5 Institute of Molecular Biotechnology, Graz University of Technology, Graz, Austria * [email protected] OPEN ACCESS Abstract Citation: Daniel B, Wallner S, Steiner B, Oberdorfer Berberine bridge enzyme-like (BBE-like) proteins form a multigene family (pfam 08031), G, Kumar P, van der Graaff E, et al. -

Dr. Duke's Phytochemical and Ethnobotanical Databases Chemicals Found in Papaver Somniferum
Dr. Duke's Phytochemical and Ethnobotanical Databases Chemicals found in Papaver somniferum Activities Count Chemical Plant Part Low PPM High PPM StdDev Refernce Citation 0 (+)-LAUDANIDINE Fruit -- 0 (+)-RETICULINE Fruit -- 0 (+)-RETICULINE Latex Exudate -- 0 (-)-ALPHA-NARCOTINE Inflorescence -- 0 (-)-NARCOTOLINE Inflorescence -- 0 (-)-SCOULERINE Latex Exudate -- 0 (-)-SCOULERINE Plant -- 0 10-HYDROXYCODEINE Latex Exudate -- 0 10-NONACOSANOL Latex Exudate Chemical Constituents of Oriental Herbs (3 diff. books) 0 13-OXOCRYPTOPINE Plant -- 0 16-HYDROXYTHEBAINE Plant -- 0 20-HYDROXY- Fruit 36.0 -- TRICOSANYLCYCLOHEXA NE 0 4-HYDROXY-BENZOIC- Pericarp -- ACID 0 4-METHYL-NONACOSANE Fruit 3.2 -- 0 5'-O- Plant -- DEMETHYLNARCOTINE 0 5-HYDROXY-3,7- Latex Exudate -- DIMETHOXYPHENANTHRE NE 0 6- Plant -- ACTEONLYDIHYDROSANG UINARINE 0 6-METHYL-CODEINE Plant Father Nature's Farmacy: The aggregate of all these three-letter citations. 0 6-METHYL-CODEINE Fruit -- 0 ACONITASE Latex Exudate -- 32 AESCULETIN Pericarp -- 3 ALANINE Seed 11780.0 12637.0 0.5273634907250652 -- Activities Count Chemical Plant Part Low PPM High PPM StdDev Refernce Citation 0 ALKALOIDS Latex Exudate 50000.0 250000.0 ANON. 1948-1976. The Wealth of India raw materials. Publications and Information Directorate, CSIR, New Delhi. 11 volumes. 5 ALLOCRYPTOPINE Plant Father Nature's Farmacy: The aggregate of all these three-letter citations. 15 ALPHA-LINOLENIC-ACID Seed 1400.0 5564.0 -0.22115561650586155 -- 2 ALPHA-NARCOTINE Plant Jeffery B. Harborne and H. Baxter, eds. 1983. Phytochemical Dictionary. A Handbook of Bioactive Compounds from Plants. Taylor & Frost, London. 791 pp. 17 APOMORPHINE Plant Father Nature's Farmacy: The aggregate of all these three-letter citations. 0 APOREINE Fruit -- 0 ARABINOSE Fruit ANON. -

Redalyc.Identification of Isoquinoline Alkaloids from Berberis Microphylla
Boletín Latinoamericano y del Caribe de Plantas Medicinales y Aromáticas ISSN: 0717-7917 [email protected] Universidad de Santiago de Chile Chile MANOSALVA, Loreto; MUTIS, Ana; DÍAZ, Juan; URZÚA, Alejandro; FAJARDO, Víctor; QUIROZ, Andrés Identification of isoquinoline alkaloids from Berberis microphylla by HPLC ESI-MS/MS Boletín Latinoamericano y del Caribe de Plantas Medicinales y Aromáticas, vol. 13, núm. 4, 2014, pp. 324-335 Universidad de Santiago de Chile Santiago, Chile Available in: http://www.redalyc.org/articulo.oa?id=85631435002 How to cite Complete issue Scientific Information System More information about this article Network of Scientific Journals from Latin America, the Caribbean, Spain and Portugal Journal's homepage in redalyc.org Non-profit academic project, developed under the open access initiative © 2014 Boletín Latinoamericano y del Caribe de Plantas Medicinales y Aromáticas 13 (4): 324 - 335 ISSN 0717 7917 www.blacpma.usach.cl Artículo Original | Original Article In memorian Professor Luis Astudillo, Universidad de Talca, Chile Identification of isoquinoline alkaloids from Berberis microphylla by HPLC ESI-MS/MS [Identificación de alcaloides isoquinolínicos en Berberis microphylla G. Forst mediante CLAE IES-MS/MS] Loreto MANOSALVA1, Ana MUTIS2, Juan DÍAZ3, Alejandro URZÚA4, Víctor FAJARDO5 & Andrés QUIROZ2 1Doctorado en Ciencias de Recursos Naturales; 2Laboratorio de Ecología Química, Departamento de Ciencias Químicas y Recursos Naturales; 3Laboratory of Mass Spectrometry, Scientific and Technological Bioresource Nucleus (Bioren), Universidad de La Frontera, Temuco, Chile 4Laboratory of Chemical Ecology, Department of Environmental Sciences, Faculty of Chemistry and Biology, Universidad de Santiago de Chile 5Chile Laboratorio de Productos Naturales, Universidad de Magallanes, Punta Arenas, Chile Contactos | Contacts: Andrés QUIROZ - E-mail address: [email protected] Abstract: Berberis microphylla (G.