The Neomuran Revolution and Phagotrophic Origin of Eukaryotes and Cilia in the Light of Intracellular Coevolution and a Revised Tree of Life
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Functional Traits and Spatio-Temporal Structure of a Major Group of Soil Protists (Rhizaria: Cercozoa) in a Temperate Grassland
fmicb-10-01332 June 8, 2019 Time: 10:19 # 1 ORIGINAL RESEARCH published: 11 June 2019 doi: 10.3389/fmicb.2019.01332 Functional Traits and Spatio-Temporal Structure of a Major Group of Soil Protists (Rhizaria: Cercozoa) in a Temperate Grassland Anna Maria Fiore-Donno1,2*, Tim Richter-Heitmann3, Florine Degrune4,5, Kenneth Dumack1,2, Kathleen M. Regan6, Sven Marhan7, Runa S. Boeddinghaus7, Matthias C. Rillig4,5, Michael W. Friedrich3, Ellen Kandeler7 and Michael Bonkowski1,2 1 Terrestrial Ecology Group, Institute of Zoology, University of Cologne, Cologne, Germany, 2 Cluster of Excellence on Plant Sciences (CEPLAS), Cologne, Germany, 3 Microbial Ecophysiology Group, Faculty of Biology/Chemistry, University of Bremen, Bremen, Germany, 4 Institute of Biology, Plant Ecology, Freie Universität Berlin, Berlin, Germany, 5 Berlin-Brandenburg Institute of Advanced Biodiversity Research, Berlin, Germany, 6 The Ecosystems Center, Marine Biological Laboratory, Woods Hole, MA, United States, 7 Department of Soil Biology, Institute of Soil Science and Land Evaluation, University of Hohenheim, Stuttgart, Germany Edited by: Soil protists are increasingly appreciated as essential components of soil foodwebs; Jaak Truu, University of Tartu, Estonia however, there is a dearth of information on the factors structuring their communities. Reviewed by: Here we investigate the importance of different biotic and abiotic factors as key Anne Winding, drivers of spatial and seasonal distribution of protistan communities. We conducted Aarhus University, Denmark 2 Ida Karlsson, an intensive survey of a 10 m grassland plot in Germany, focusing on a major group Swedish University of Agricultural of protists, the Cercozoa. From 177 soil samples, collected from April to November, Sciences, Sweden we obtained 694 Operational Taxonomy Units representing 6 million Illumina reads. -

Download This Publication (PDF File)
PUBLIC LIBRARY of SCIENCE | plosgenetics.org | ISSN 1553-7390 | Volume 2 | Issue 12 | DECEMBER 2006 GENETICS PUBLIC LIBRARY of SCIENCE www.plosgenetics.org Volume 2 | Issue 12 | DECEMBER 2006 Interview Review Knight in Common Armor: 1949 Unraveling the Genetics 1956 An Interview with Sir John Sulston e225 of Human Obesity e188 Jane Gitschier David M. Mutch, Karine Clément Research Articles Natural Variants of AtHKT1 1964 The Complete Genome 2039 Enhance Na+ Accumulation e210 Sequence and Comparative e206 in Two Wild Populations of Genome Analysis of the High Arabidopsis Pathogenicity Yersinia Ana Rus, Ivan Baxter, enterocolitica Strain 8081 Balasubramaniam Muthukumar, Nicholas R. Thomson, Sarah Jeff Gustin, Brett Lahner, Elena Howard, Brendan W. Wren, Yakubova, David E. Salt Matthew T. G. Holden, Lisa Crossman, Gregory L. Challis, About the Cover Drosophila SPF45: A Bifunctional 1974 Carol Churcher, Karen The jigsaw image of representatives Protein with Roles in Both e178 Mungall, Karen Brooks, Tracey of various lines of eukaryote evolution Splicing and DNA Repair Chillingworth, Theresa Feltwell, refl ects the current lack of consensus as Ahmad Sami Chaouki, Helen K. Zahra Abdellah, Heidi Hauser, to how the major branches of eukaryotes Salz Kay Jagels, Mark Maddison, fi t together. The illustrations from upper Sharon Moule, Mandy Sanders, left to bottom right are as follows: a single Mammalian Small Nucleolar 1984 Sally Whitehead, Michael A. scale from the surface of Umbellosphaera; RNAs Are Mobile Genetic e205 Quail, Gordon Dougan, Julian Amoeba, the large amoeboid organism Elements Parkhill, Michael B. Prentice used as an introduction to protists for Michel J. Weber many school children; Euglena, the iconic Low Levels of Genetic 2052 fl agellate that is often used to challenge Soft Sweeps III: The Signature 1998 Divergence across e215 ideas of plants (Euglena has chloroplasts) of Positive Selection from e186 Geographically and and animals (Euglena moves); Stentor, Recurrent Mutation Linguistically Diverse one of the larger ciliates; Cacatua, the Pleuni S. -

Microbiology Unit- 1 1. According to Bergey's Manual of Systematic
Microbiology Unit- 1 1. According to Bergey’s Manual of Systematic Bacteriology, prokaryotes that lack a cell wall belong to the group? a) Gracilicutes b) Firmicutes c) Tenericutes d) Mendosicutes 2.Which among the following kingdoms were proposed by Whittaker? a) Monera b) Protista,Fungi c) Plantae,Animalia d) Monera,Protista,Fungi,Plantae,Animalia 3. Lipopolysaccharide in cell walls is characteristic of? a) Gram-positive bacteria b) Gram-negative bacteria c) Fungi d) Algae 4. Which of the following symmetry is exhibited by rod-shaped viruses? a) icosahedral b) helical c) complex d) circular 5. Fungi are ______________ a) prokaryotic b) eukaryotic c) prokaryotic and lack chlorophyll d) eukaryotic and lack chlorophyll 6. Which microorganism(s) among the following perform photosynthesis by utilizing light? a) Cyanobacteria b) Fungi c) Viruses d) Cyanobacteria, Fungi and Viruses 7. Adenoviruses exhibit which of the following symmetry? a) helical symmetry b) circular symmetry c) icosahedral symmetry d) complex structure symmetry 8. The transfer of genes from one cell to another by a bacteriophage is known as __________________ a) Recombination b) Conjugation c) Transduction d) Transformation 9. The cell in which the F factor carries along with it some chromosomal genes are known as ____________ a) F+ cell b) F— cell c) F’ cell d) F’’’ cell 10. The xanthophyte walls are typically of _____________________ a) chitin b) cellulose c) cellulose and pectin d) starch 11. Vaucheria is a single-celled organism. a) True b) False 12. In Chlamydomonas the most common method of sexual reproduction is ________________ a) isogamy b) heterogamy c) oogamy d) spore formation 13. -

Functional Replacement of a Primary Metabolic Pathway Via Multiple Independent Eukaryote-To-Eukaryote Gene Transfers and Selective Retention
doi: 10.1111/j.1420-9101.2009.01797.x Functional replacement of a primary metabolic pathway via multiple independent eukaryote-to-eukaryote gene transfers and selective retention A. M. NEDELCU, A. J. C. BLAKNEY & K. D. LOGUE Biology Department, University of New Brunswick, Fredericton, NB, Canada Keywords: Abstract ammonium transporter; Although lateral gene transfer (LGT) is now recognized as a major force in the glutamate synthase; evolution of prokaryotes, the contribution of LGT to the evolution and glutamine synthetase; diversification of eukaryotes is less understood. Notably, transfers of complete lateral gene transfer; pathways are believed to be less likely between eukaryotes, because the Monosiga; successful transfer of a pathway requires the physical clustering of functionally primary metabolic pathway. related genes. Here, we report that in one of the closest unicellular relatives of animals, the choanoflagellate, Monosiga, three genes whose products work together in the glutamate synthase cycle are of algal origin. The concerted retention of these three independently acquired genes is best explained as the consequence of a series of adaptive replacement events. More generally, this study argues that (i) eukaryote-to-eukaryote transfers of entire metabolic pathways are possible, (ii) adaptive functional replacements of primary pathways can occur, and (iii) functional replacements involving eukaryotic genes are likely to have also contributed to the evolution of eukaryotes. Lastly, these data underscore the potential contribution of algal genes to the evolution of nonphotosynthetic lineages. Palmer, 2008). On the other hand, as functional replace- Introduction ments can be either adaptive or selectively neutral, their Lateral gene transfer (LGT) is currently recognized as a impact on the recipient’s adaptive or long-term evolu- major force in the evolution of prokaryotes (e.g. -

The Phagotrophic Origin of Eukaryotes and Phylogenetic Classification Of
International Journal of Systematic and Evolutionary Microbiology (2002), 52, 297–354 DOI: 10.1099/ijs.0.02058-0 The phagotrophic origin of eukaryotes and phylogenetic classification of Protozoa Department of Zoology, T. Cavalier-Smith University of Oxford, South Parks Road, Oxford OX1 3PS, UK Tel: j44 1865 281065. Fax: j44 1865 281310. e-mail: tom.cavalier-smith!zoo.ox.ac.uk Eukaryotes and archaebacteria form the clade neomura and are sisters, as shown decisively by genes fragmented only in archaebacteria and by many sequence trees. This sisterhood refutes all theories that eukaryotes originated by merging an archaebacterium and an α-proteobacterium, which also fail to account for numerous features shared specifically by eukaryotes and actinobacteria. I revise the phagotrophy theory of eukaryote origins by arguing that the essentially autogenous origins of most eukaryotic cell properties (phagotrophy, endomembrane system including peroxisomes, cytoskeleton, nucleus, mitosis and sex) partially overlapped and were synergistic with the symbiogenetic origin of mitochondria from an α-proteobacterium. These radical innovations occurred in a derivative of the neomuran common ancestor, which itself had evolved immediately prior to the divergence of eukaryotes and archaebacteria by drastic alterations to its eubacterial ancestor, an actinobacterial posibacterium able to make sterols, by replacing murein peptidoglycan by N-linked glycoproteins and a multitude of other shared neomuran novelties. The conversion of the rigid neomuran wall into a flexible surface coat and the associated origin of phagotrophy were instrumental in the evolution of the endomembrane system, cytoskeleton, nuclear organization and division and sexual life-cycles. Cilia evolved not by symbiogenesis but by autogenous specialization of the cytoskeleton. -

A Revised Classification of Naked Lobose Amoebae (Amoebozoa
Protist, Vol. 162, 545–570, October 2011 http://www.elsevier.de/protis Published online date 28 July 2011 PROTIST NEWS A Revised Classification of Naked Lobose Amoebae (Amoebozoa: Lobosa) Introduction together constitute the amoebozoan subphy- lum Lobosa, which never have cilia or flagella, Molecular evidence and an associated reevaluation whereas Variosea (as here revised) together with of morphology have recently considerably revised Mycetozoa and Archamoebea are now grouped our views on relationships among the higher-level as the subphylum Conosa, whose constituent groups of amoebae. First of all, establishing the lineages either have cilia or flagella or have lost phylum Amoebozoa grouped all lobose amoe- them secondarily (Cavalier-Smith 1998, 2009). boid protists, whether naked or testate, aerobic Figure 1 is a schematic tree showing amoebozoan or anaerobic, with the Mycetozoa and Archamoe- relationships deduced from both morphology and bea (Cavalier-Smith 1998), and separated them DNA sequences. from both the heterolobosean amoebae (Page and The first attempt to construct a congruent molec- Blanton 1985), now belonging in the phylum Per- ular and morphological system of Amoebozoa by colozoa - Cavalier-Smith and Nikolaev (2008), and Cavalier-Smith et al. (2004) was limited by the the filose amoebae that belong in other phyla lack of molecular data for many amoeboid taxa, (notably Cercozoa: Bass et al. 2009a; Howe et al. which were therefore classified solely on morpho- 2011). logical evidence. Smirnov et al. (2005) suggested The phylum Amoebozoa consists of naked and another system for naked lobose amoebae only; testate lobose amoebae (e.g. Amoeba, Vannella, this left taxa with no molecular data incertae sedis, Hartmannella, Acanthamoeba, Arcella, Difflugia), which limited its utility. -
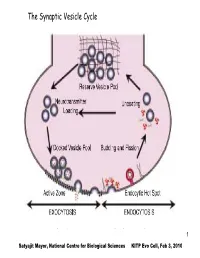
The Synaptic Vesicle Cycle
The Synaptic Vesicle Cycle 1 Satyajit Mayor, National Centre for Biological Sciences KITP Evo Cell, Feb 3, 2010 The Eukaryotic Commandments: T. Cavalier-Smith (i)origin of the endomembrane system (ER, Golgi and lysosomes) and coated- vesicle budding and fusion, including endocytosis and exocytosis; (ii) origin of the cytoskeleton, centrioles, cilia and associated molecular motors; (iii) origin of the nucleus, nuclear pore complex and trans-envelope protein and RNA transport; (iv) origin of linear chromosomes with plural replicons, centromeres and telomeres; (v) origin of novel cell-cycle controls and mitotic segregation; (vi) origin of sex (syngamy, nuclear fusion and meiosis); (vii) origin of peroxisomes; (ix) origin of mitochondria; (viii) novel patterns of rRNAprocessing using small nucleolar-ribonucleoproteins (snoRNPs); (x) origin of spliceosomal introns. 2 EM of Chemical synapse mitochondria Active Zone Figure 5.3, Bear, 2001 3 T. Cavalier-Smith International Journal of Systematic and Evolutionary Microbiology (2002), 52, 297–354 Fig. 1. The bacterial origins of eukaryotes as a two-stage process. I phase: The ancestors of eukaryotes, the stem neomura, are shared with archaebacteria and evolved during the neomuran revolution, in which N-linked glycoproteins replaced murein peptidoglycan and 18 other suites of characters changed radically through adaptation of an ancestral actinobacterium to thermophily, as discussed in detail by Cavalier- Smith (2002a). 4 T. Cavalier-Smith International Journal of Systematic and Evolutionary Microbiology (2002), 52, 297–354 Fig. 1. The bacterial origins of eukaryotes as a two-stage process. II phase: In the next phase, archaebacteria and eukaryotes diverged dramatically. Archaebacteria retained the wall and therefore their general bacterial cell and genetic organization, but became adapted to even hotter and more acidic environments by substituting prenyl ether lipids for the ancestral acyl esters and making new acid- resistant flagellar shafts (Cavalier-Smith, 2002a). -

What Is Macroevolution?
[Palaeontology, 2020, pp. 1–11] FRONTIERS IN PALAEONTOLOGY WHAT IS MACROEVOLUTION? by MICHAEL HAUTMANN Pal€aontologisches Institut und Museum, Universit€at Zurich,€ Karl-Schmid Strasse 4, 8006 Zurich,€ Switzerland; [email protected] Typescript received 14 June 2019; accepted in revised form 15 October 2019 Abstract: Definitions of macroevolution fall into three cat- intraspecific competition as a mediator between selective egories: (1) evolution of taxa of supraspecific rank; (2) evolu- agents and evolutionary responses. This mediating role of tion on the grand time-scale; and (3) evolution that is guided intraspecific competition occurs in the presence of sexual by sorting of interspecific variation (as opposed to sorting of reproduction and has therefore no analogue at the macroevo- intraspecific variation in microevolution). Here, it is argued lutionary level where species are the evolutionary units. Com- that only definition 3 allows for a consistent separation of petition between species manifests both on the macroevolution and microevolution. Using this definition, spe- microevolutionary and macroevolutionary level, but with dif- ciation has both microevolutionary and macroevolutionary ferent effects. In microevolution, interspecific competition aspects: the process of morphological transformation is spurs evolutionary divergence, whereas it is a potential driver microevolutionary, but the variation among species that it pro- of extinction at the macroevolutionary level. Recasting the Red duces is macroevolutionary, as is the rate at which speciation Queen hypothesis in a macroevolutionary framework suggests occurs. Selective agents may have differential effects on that the effects of interspecific competition result in a positive intraspecific and interspecific variation, with three possible sit- correlation between origination and extinction rates, confirm- uations: effect at one level only, effect at both levels with the ing empirical observations herein referred to as Stanley’s rule. -

Chapter 11: Bacteria Bacterial Groups
Bacterial Groups u Most widely accepted taxonomic classification for bacteria is Bergey’s Manual of Systematic Bacteriology. u 5000 bacterial species identified, 3100 classified. Chapter 11: Bacteria u Bacteria are divided into four divisions (phyla) according to the characteristics of their cell walls. u Each division is divided into sections according to: u Gram stain reaction u Cell shape u Cell arrangements u Oxygen requirements u Motility u Nutritional and metabolic properties u Each section contains several genera. Four Divisions of Bacteria Classification of Bacteria Procaryotes Gram-Negative Division II Wall-Less Archaea Bacteria Bacteria Bacteria Bacteria (Gracilicutes) (Firmicutes) (Tenericutes) (Mendosicutes) Thin Cell Walls Thick cell Walls Lack cell walls Unusual cell walls Division I. Gram-Negative Bacteria Gram Negative Bacteria Spirochetes 1. Spirochetes u Helical shape. Flexible. u Contain two or more axial filaments (endoflagella). u Move in corkscrew pattern. u Medically important members: F Treponema pallidum: Syphilis F Borrelia spp.: Lyme disease, relapsing fever F Leptospira: Leptospirosis 1 Syphilis is Caused by a Spirochete Lyme Disease is Caused by a Spirochete Primary syphilitic chancre and secondary rash. Source: Tropical Medicine and Parasitology, 1997 Lyme Disease early lesion at tick bite site. Source: Medical Microbiology, 1998 2. Aerobic, Motile, Helical/Vibroid Gram- Negative Bacteria Gram Negative Bacteria u Rigid helical shape or curved rods. Aerobic, Motile, Helical/Vibroid u Lack axial filaments (endoflagella); have polar Gram-Negative Bacteria flagella instead. u Most are harmless aquatic organisms. u Genus Azospirillum fixes nitrogen in soil. u Genus Bdellovibrio attacks other bacteria. u Important pathogens include: F Campylobacter jejuni: Most common bacterial food- borne intestinal disease in the United States (2 million cases/year). -

Card Uses a Minor Groove Wedge Mechanism to Stabilize the RNA
1 CarD uses a minor groove wedge mechanism to stabilize the RNA 2 polymerase open promoter complex 3 4 Brian Bae1, James Chen1, Elizabeth Davis1, Katherine Leon1, Seth A. Darst1,*, 5 Elizabeth A. Campbell1,* 6 7 1The Rockefeller University, Laboratory for Molecular Biophysics, 1230 York Avenue, New York, 8 NY 10065, USA. 9 10 *Correspondence to: E-mail: [email protected], [email protected] 11 12 Present Address: Elizabeth Davis, The University of Minnesota School of Medicine, 13 420 Delaware St. SE, Minneapolis, MN 55455, USA; Katherine Leon, Department of 14 Biochemistry and Molecular Biology, University of Chicago, 929 East 57th Street, GCIS 15 W219 Chicago, IL 60637, USA. 16 17 2 18 Abstract A key point to regulate gene expression is at transcription initiation, and 19 activators play a major role. CarD, an essential activator in Mycobacterium tuberculosis, 20 is found in many bacteria, including Thermus species, but absent in Escherichia coli. To 21 delineate the molecular mechanism of CarD, we determined crystal structures of 22 Thermus transcription initiation complexes containing CarD. The structures show CarD 23 interacts with the unique DNA topology presented by the upstream double- 24 stranded/single-stranded DNA junction of the transcription bubble. We confirm that our 25 structures correspond to functional activation complexes, and extend our understanding 26 of the role of a conserved CarD Trp residue that serves as a minor groove wedge, 27 preventing collapse of the transcription bubble to stabilize the transcription initiation 28 complex. Unlike E. coli RNAP, many bacterial RNAPs form unstable promoter 29 complexes, explaining the need for CarD. -

The Apicoplast: a Review of the Derived Plastid of Apicomplexan Parasites
Curr. Issues Mol. Biol. 7: 57-80. Online journalThe Apicoplastat www.cimb.org 57 The Apicoplast: A Review of the Derived Plastid of Apicomplexan Parasites Ross F. Waller1 and Geoffrey I. McFadden2,* way to apicoplast discovery with studies of extra- chromosomal DNAs recovered from isopycnic density 1Botany, University of British Columbia, 3529-6270 gradient fractionation of total Plasmodium DNA. This University Boulevard, Vancouver, BC, V6T 1Z4, Canada group recovered two DNA forms; one a 6kb tandemly 2Plant Cell Biology Research Centre, Botany, University repeated element that was later identifed as the of Melbourne, 3010, Australia mitochondrial genome, and a second, 35kb circle that was supposed to represent the DNA circles previously observed by microscopists (Wilson et al., 1996b; Wilson Abstract and Williamson, 1997). This molecule was also thought The apicoplast is a plastid organelle, homologous to to be mitochondrial DNA, and early sequence data of chloroplasts of plants, that is found in apicomplexan eubacterial-like rRNA genes supported this organellar parasites such as the causative agents of Malaria conclusion. However, as the sequencing effort continued Plasmodium spp. It occurs throughout the Apicomplexa a new conclusion, that was originally embraced with and is an ancient feature of this group acquired by the some awkwardness (“Have malaria parasites three process of endosymbiosis. Like plant chloroplasts, genomes?”, Wilson et al., 1991), began to emerge. apicoplasts are semi-autonomous with their own genome Gradually, evermore convincing character traits of a and expression machinery. In addition, apicoplasts import plastid genome were uncovered, and strong parallels numerous proteins encoded by nuclear genes. These with plastid genomes from non-photosynthetic plants nuclear genes largely derive from the endosymbiont (Epifagus virginiana) and algae (Astasia longa) became through a process of intracellular gene relocation. -

And Thermo-Adaptation in Hyperthermophilic Archaea: Identification of Compatible Solutes, Accumulation Profiles, and Biosynthetic Routes in Archaeoglobus Spp
Universidade Nova de Lisboa Osmo- andInstituto thermo de Tecnologia-adaptation Química e Biológica in hyperthermophilic Archaea: Subtitle Subtitle Luís Pedro Gafeira Gonçalves Osmo- and thermo-adaptation in hyperthermophilic Archaea: identification of compatible solutes, accumulation profiles, and biosynthetic routes in Archaeoglobus spp. OH OH OH CDP c c c - CMP O O - PPi O3P P CTP O O O OH OH OH OH OH OH O- C C C O P O O P i Dissertation presented to obtain the Ph.D degree in BiochemistryO O- Instituto de Tecnologia Química e Biológica | Universidade Nova de LisboaP OH O O OH OH OH Oeiras, Luís Pedro Gafeira Gonçalves January, 2008 2008 Universidade Nova de Lisboa Instituto de Tecnologia Química e Biológica Osmo- and thermo-adaptation in hyperthermophilic Archaea: identification of compatible solutes, accumulation profiles, and biosynthetic routes in Archaeoglobus spp. This dissertation was presented to obtain a Ph. D. degree in Biochemistry at the Instituto de Tecnologia Química e Biológica, Universidade Nova de Lisboa. By Luís Pedro Gafeira Gonçalves Supervised by Prof. Dr. Helena Santos Oeiras, January, 2008 Apoio financeiro da Fundação para a Ciência e Tecnologia (POCI 2010 – Formação Avançada para a Ciência – Medida IV.3) e FSE no âmbito do Quadro Comunitário de apoio, Bolsa de Doutoramento com a referência SFRH / BD / 5076 / 2001. ii ACKNOWNLEDGMENTS The work presented in this thesis, would not have been possible without the help, in terms of time and knowledge, of many people, to whom I am extremely grateful. Firstly and mostly, I need to thank my supervisor, Prof. Helena Santos, for her way of thinking science, her knowledge, her rigorous criticism, and her commitment to science.