Microbiology Unit- 1 1. According to Bergey's Manual of Systematic
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Chapter 11: Bacteria Bacterial Groups
Bacterial Groups u Most widely accepted taxonomic classification for bacteria is Bergey’s Manual of Systematic Bacteriology. u 5000 bacterial species identified, 3100 classified. Chapter 11: Bacteria u Bacteria are divided into four divisions (phyla) according to the characteristics of their cell walls. u Each division is divided into sections according to: u Gram stain reaction u Cell shape u Cell arrangements u Oxygen requirements u Motility u Nutritional and metabolic properties u Each section contains several genera. Four Divisions of Bacteria Classification of Bacteria Procaryotes Gram-Negative Division II Wall-Less Archaea Bacteria Bacteria Bacteria Bacteria (Gracilicutes) (Firmicutes) (Tenericutes) (Mendosicutes) Thin Cell Walls Thick cell Walls Lack cell walls Unusual cell walls Division I. Gram-Negative Bacteria Gram Negative Bacteria Spirochetes 1. Spirochetes u Helical shape. Flexible. u Contain two or more axial filaments (endoflagella). u Move in corkscrew pattern. u Medically important members: F Treponema pallidum: Syphilis F Borrelia spp.: Lyme disease, relapsing fever F Leptospira: Leptospirosis 1 Syphilis is Caused by a Spirochete Lyme Disease is Caused by a Spirochete Primary syphilitic chancre and secondary rash. Source: Tropical Medicine and Parasitology, 1997 Lyme Disease early lesion at tick bite site. Source: Medical Microbiology, 1998 2. Aerobic, Motile, Helical/Vibroid Gram- Negative Bacteria Gram Negative Bacteria u Rigid helical shape or curved rods. Aerobic, Motile, Helical/Vibroid u Lack axial filaments (endoflagella); have polar Gram-Negative Bacteria flagella instead. u Most are harmless aquatic organisms. u Genus Azospirillum fixes nitrogen in soil. u Genus Bdellovibrio attacks other bacteria. u Important pathogens include: F Campylobacter jejuni: Most common bacterial food- borne intestinal disease in the United States (2 million cases/year). -

Sepf Is the Ftsz Anchor in Archaea, with Features of an Ancestral Cell Division System
ARTICLE https://doi.org/10.1038/s41467-021-23099-8 OPEN SepF is the FtsZ anchor in archaea, with features of an ancestral cell division system Nika Pende1,8, Adrià Sogues2,8, Daniela Megrian1,3, Anna Sartori-Rupp4, Patrick England 5, Hayk Palabikyan6, ✉ Simon K.-M. R. Rittmann 6, Martín Graña 7, Anne Marie Wehenkel 2 , Pedro M. Alzari 2 & ✉ Simonetta Gribaldo 1 Most archaea divide by binary fission using an FtsZ-based system similar to that of bacteria, 1234567890():,; but they lack many of the divisome components described in model bacterial organisms. Notably, among the multiple factors that tether FtsZ to the membrane during bacterial cell constriction, archaea only possess SepF-like homologs. Here, we combine structural, cellular, and evolutionary analyses to demonstrate that SepF is the FtsZ anchor in the human-associated archaeon Methanobrevibacter smithii. 3D super-resolution microscopy and quantitative analysis of immunolabeled cells show that SepF transiently co-localizes with FtsZ at the septum and possibly primes the future division plane. M. smithii SepF binds to membranes and to FtsZ, inducing filament bundling. High-resolution crystal structures of archaeal SepF alone and in complex with the FtsZ C-terminal domain (FtsZCTD) reveal that SepF forms a dimer with a homodimerization interface driving a binding mode that is different from that previously reported in bacteria. Phylogenetic analyses of SepF and FtsZ from bacteria and archaea indicate that the two proteins may date back to the Last Universal Common Ancestor (LUCA), and we speculate that the archaeal mode of SepF/FtsZ interaction might reflect an ancestral feature. Our results provide insights into the mechanisms of archaeal cell division and pave the way for a better understanding of the processes underlying the divide between the two prokaryotic domains. -

General Microbiology Appliance
• Ministry of Health, Republic of Belarus Institution of Education “Grodno State Medical University” Department of Microbiology, Virology and Immunology named after S.I.Gelberg GENERAL MICROBIOLOGY Training appliance for students of the Department for International Students The Subject of Microbiology Microscopic Method of Investigation and Staining Techniques Theme No1 DEFINITION OF THE TERMS “MICROBIOLOGY” AND “MICROORGANISM” • Science studying microorganisms. • Organisms, invisible by the unaided eye (microscopic object = microbe) CLASSIFICATION OF MICROBIOLOGICAL SCIENCES • According to the topic (object) of research - General microbiology - Individual microbiological sciences • bacteriology (prokaryotes) • mycology (eukaryotes-fungi) • protozoology (eukaryotes - multicellular parasites) • virology (viruses) • According to their application • medical • sanitary • veterinary • industrial • soil • sea • space CLASSIFICATION OF MICROBIOLOGICAL SCIENCES According to the topic According to their (object) of research application General microbiology • medical • sanitary Individual microbiological sciences: • veterinary • bacteriology • industrial (prokaryotes) • soil • mycology (eukaryotes- fungi) • sea • protozoology • space (eukaryotes - multicellular parasites) • virology (viruses) TASKS OF MEDICAL MICROBIOLOGY • Study of structure and biological properties of microorganisms • Study of cointeraction of microorganism with human organism (i.e. infection), namely: - pathogenesis - diagnostics - treatment - preventive maintenance MICROBIOLOGICAL -

Taxonomic Hierarchy of the Phylum Proteobacteria and Korean Indigenous Novel Proteobacteria Species
Journal of Species Research 8(2):197-214, 2019 Taxonomic hierarchy of the phylum Proteobacteria and Korean indigenous novel Proteobacteria species Chi Nam Seong1,*, Mi Sun Kim1, Joo Won Kang1 and Hee-Moon Park2 1Department of Biology, College of Life Science and Natural Resources, Sunchon National University, Suncheon 57922, Republic of Korea 2Department of Microbiology & Molecular Biology, College of Bioscience and Biotechnology, Chungnam National University, Daejeon 34134, Republic of Korea *Correspondent: [email protected] The taxonomic hierarchy of the phylum Proteobacteria was assessed, after which the isolation and classification state of Proteobacteria species with valid names for Korean indigenous isolates were studied. The hierarchical taxonomic system of the phylum Proteobacteria began in 1809 when the genus Polyangium was first reported and has been generally adopted from 2001 based on the road map of Bergey’s Manual of Systematic Bacteriology. Until February 2018, the phylum Proteobacteria consisted of eight classes, 44 orders, 120 families, and more than 1,000 genera. Proteobacteria species isolated from various environments in Korea have been reported since 1999, and 644 species have been approved as of February 2018. In this study, all novel Proteobacteria species from Korean environments were affiliated with four classes, 25 orders, 65 families, and 261 genera. A total of 304 species belonged to the class Alphaproteobacteria, 257 species to the class Gammaproteobacteria, 82 species to the class Betaproteobacteria, and one species to the class Epsilonproteobacteria. The predominant orders were Rhodobacterales, Sphingomonadales, Burkholderiales, Lysobacterales and Alteromonadales. The most diverse and greatest number of novel Proteobacteria species were isolated from marine environments. Proteobacteria species were isolated from the whole territory of Korea, with especially large numbers from the regions of Chungnam/Daejeon, Gyeonggi/Seoul/Incheon, and Jeonnam/Gwangju. -

A Metagenomics Roadmap to the Uncultured Genome Diversity in Hypersaline Soda Lake Sediments Charlotte D
Vavourakis et al. Microbiome (2018) 6:168 https://doi.org/10.1186/s40168-018-0548-7 RESEARCH Open Access A metagenomics roadmap to the uncultured genome diversity in hypersaline soda lake sediments Charlotte D. Vavourakis1 , Adrian-Stefan Andrei2†, Maliheh Mehrshad2†, Rohit Ghai2, Dimitry Y. Sorokin3,4 and Gerard Muyzer1* Abstract Background: Hypersaline soda lakes are characterized by extreme high soluble carbonate alkalinity. Despite the high pH and salt content, highly diverse microbial communities are known to be present in soda lake brines but the microbiome of soda lake sediments received much less attention of microbiologists. Here, we performed metagenomic sequencing on soda lake sediments to give the first extensive overview of the taxonomic diversity found in these complex, extreme environments and to gain novel physiological insights into the most abundant, uncultured prokaryote lineages. Results: We sequenced five metagenomes obtained from four surface sediments of Siberian soda lakes with a pH 10 and a salt content between 70 and 400 g L−1. The recovered 16S rRNA gene sequences were mostly from Bacteria,evenin the salt-saturated lakes. Most OTUs were assigned to uncultured families. We reconstructed 871 metagenome-assembled genomes (MAGs) spanning more than 45 phyla and discovered the first extremophilic members of the Candidate Phyla Radiation (CPR). Five new species of CPR were among the most dominant community members. Novel dominant lineages were found within previously well-characterized functional groups involved in carbon, sulfur, and nitrogen cycling. Moreover, key enzymes of the Wood-Ljungdahl pathway were encoded within at least four bacterial phyla never previously associated with this ancient anaerobic pathway for carbon fixation and dissimilation, including the Actinobacteria. -

Antibacterial Activity of Thin Films Tio Doped with Ag And
BioDiscovery 20: e15076 doi: 10.3897/biodiscovery.20.e15076 Conference Abstract Antibacterial activity of thin films TiO2 doped with Ag and Cu on Gracilicutes and Firmicutes bacteria Dragomira S. Stoyanova‡, Iliana A. Ivanova‡§, Orlin I. Angelov , Todorka G. Vladkova| ‡ Sofia University “St. Kl. Ohridski”, Sofia, Bulgaria § Bulgarian Academy of Sciences, Sofia, Bulgaria | University of Chemical Technology and Metallurgy, Sofia, Bulgaria Corresponding author: Dragomira S. Stoyanova ([email protected]) Received: 14 Jul 2017 | Published: 17 Jul 2017 Citation: Stoyanova D, Ivanova I, Angelov O, Vladkova T (2017) Antibacterial activity of thin films TiO2 doped with Ag and Cu on Gracilicutes and Firmicutes bacteria. BioDiscovery 20: e15076. https://doi.org/10.3897/biodiscovery.20.e15076 Abstract This research aims to study the antibacterial activity of thin films nanostructured TiO 2 doped with Ag and Cu on Gracilicutes and Firmicutes bacteria with clinical significance. The thin films were deposited on glass substrates without heating during the deposition by radio frequency magnetron co-sputtering of TiO2 target and pieces of Ag and Cu. The total surface area of Ag was 60 mm2 and this one of Cu was 100 mm2 . The r.f. power was 50W and sputtering atmosphere was Ar (0,8 Pa). The thickness of the films was about 60 nm. The experiment was conducted under day light regime. The test strains Bacillus cereus, Staphylococcus epidermidis, Salmonella enterica, Escherichia coli and Pseudomonas sp. were used. The bactericidal effect was established at different time point between 30 min - 90 min for Pseudomonas sp. and S. enterica. The Firmicutes bacteria B. cereus and S. -

The Basic Structures of Bacteria Anatomy and Physiology
The Basic Structures of Bacteria Anatomy and Physiology ASSIST. PROF. Dr. Abdulameer Abdullah University of Basra, College of Nursing 2017-2018 2 Anatomy and Physiology of Bacteria 3 Bacterial Cell Wall Four groups based on cell wall composition 1. Gram positive cells 2. Gram negative cells 3. Bacteria without cell walls 4. Bacteria with chemically unique cell walls 4 Peptidoglycan • unique macromolecule composed of a repeating framework of long glycan chains cross-linked by short peptide fragments • provides strong, flexible support to keep bacteria from bursting or collapsing because of changes in osmotic pressure 5 Gram positive Gram negative 11 Gram-Positive & Gram-Negative cell wall Gram _____+_____ Gram _____-_____ Peptidoglycan is the thick, outermost layer of The cell walls of gram-negative bacteria are the cell wall. more chemically complex, thinner and less compact. About 90% of the Gram-positive cell wall is Peptidoglycan only 5 – 20% of the cell wall. made of peptidoglycan. Peptidoglycan is not the outermost layer, but Consists of between the plasma membrane and the a thick, homogenous sheath of outer membrane. peptidoglycan 20-80 nm thick Not accessible to the action of antibiotics. tightly bound acidic polysaccharides, Outer membrane is similar to the plasma including teichoic acid and lipoteichoic membrane, but is less permeable and acid contains lipopolysaccharides (LPS). cell membrane Retain crystal violet and stain purple LPS is a harmful substance classified as an endotoxin. Consists of an outer membrane containing lipopolysaccharide (LPS) thin shell of peptidoglycan periplasmic space inner membrane Lose crystal violet and stain red from safranin counterstain 7 Gram positive wall Gram negative cell wall 8w`1 9 Flagellum • Flagellum : Specialized appendage attached to the cell by a basal body that holds a long rotating filament. -

Proposal to Include the Rank of Phylum in the International Code of Nomenclature of Prokaryotes to Be Open for Discussion In
Proposal to include the rank of phylum in the International Code of Nomenclature of Prokaryotes To be open for discussion in the period November 1, 2020 – January 31, 2021 and ICSP ballot – February 1-28, 2021. Background information: 1. The word ‘phylum’ is not found in the ICNP (2008 revision, Parker et al. 2019) or in earlier versions of the ICNB. The highest taxon covered by the code is the class (except for the word ‘kingdom’ mentioned once in Rule 8). 2. The Approved Lists of 1980 include two ‘divisions’: the Firmacutes (sic) and the Gracilicutes; both names were derived from Gibbons and Murray (1978) Proposals concerning the higher taxa of Bacteria. Int. J. Syst. Bacteriol. 28: 1-6. Note that in the International Code of Nomenclature for algae, fungi, and plants, the term ‘division’ is equivalent to ‘phylum’. 3. The word ‘phylum’ first occurs in the prokaryotic systematics literature in a paper by Woese et al. (1985) A phylogenetic definition of the major eubacterial taxa, Syst. Appl. Microbiol. 6: 143-151. Ten groupings were proposed that ‘are appropriately termed eubacterial Phyla or Divisions’. 4. Since then the term ‘phylum’ has become extensively used and more phylum- level taxa have been proposed. Currently, LPSN lists 39 phylum-level taxa with cultivated representatives (https://www.bacterio.net/domain). However, phylum names cannot be validly published as long as the rank of phylum is not included in the rules of the ICNP. 5. The Judicial Commission of the ICSB at its meetings in Sydney in 1999 discussed the issue of taxa of higher rank and felt that discussion on this subject must be postponed until an ad hoc committee on this matter made its report. -
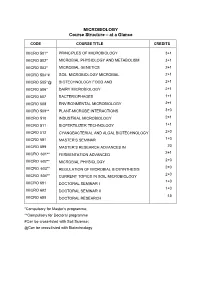
MICROBIOLOGY Course Structure – at a Glance
MICROBIOLOGY Course Structure – at a Glance CODE COURSE TITLE CREDITS MICRO 501* PRINCIPLES OF MICROBIOLOGY 3+1 MICRO 502* MICROBIAL PHYSIOLOGY AND METABOLISM 3+1 MICRO 503* MICROBIAL GENETICS 2+1 MICRO 504*# SOIL MICROBIOLOGY MICROBIAL 2+1 MICRO 505*@ BIOTECHNOLOGY FOOD AND 2+1 MICRO 506* DAIRY MICROBIOLOGY 2+1 MICRO 507 BACTERIOPHAGES 1+1 MICRO 508 ENVIRONMENTAL MICROBIOLOGY 2+1 MICRO 509** PLANT-MICROBE INTERACTIONS 3+0 MICRO 510 INDUSTRIAL MICROBIOLOGY 2+1 MICRO 511 BIOFERTILIZER TECHNOLOGY 1+1 MICRO 512 CYANOBACTERIAL AND ALGAL BIOTECHNOLOGY 2+0 MICRO 591 MASTER’S SEMINAR 1+0 MICRO 599 MASTER’S RESEARCH ADVANCES IN 20 MICRO 601** 2+1 FERMENTATION ADVANCED MICRO 602** 2+0 MICROBIAL PHYSIOLOGY MICRO 603** 2+0 REGULATION OF MICROBIAL BIOSYNTHESIS 2+0 MICRO 604** CURRENT TOPICS IN SOIL MICROBIOLOGY 1+0 MICRO 691 DOCTORAL SEMINAR I 1+0 MICRO 692 DOCTORAL SEMINAR II 45 MICRO 699 DOCTORAL RESEARCH *Compulsory for Master’s programme; **Compulsory for Doctoral programme #Can be cross-listed with Soil Science; @Can be cross-listed with Biotechnology Minor Departments 9 Plant Molecular Biology and Biotechnology Biochemistry Soil Science Plant Pathology Entomology Supporting Deparments 5 Statistics and Mathematics Plant Molecular Biology and Biotechnology Biochemistry Soil Science Plant Pathology Entomology Non credit compulsory courses CODE COURSE TITLE CREDITS PGS 501 LIBRARY AND INFORMATION SERVICES 0+1 PGS 502 TECHNICAL WRITING AND COMMUNICATION SKILLS 0+1 INTELLECTUAL PROPERTY AND ITS PGS 503 (e-course) MANAGEMENT IN AGRICULTURE 1+0 PGS 504 BASIC CONCEPTS IN LABORATORY TECHNIQUES 0+1 PGS 505 AGRICULTURAL RESEARCH, RESEARCH (e-course) ETHICS AND RURAL DEVELOPMENT PROGRAMMES 1+0 PGS 506 DISASTER MANAGEMENT 1+0 (e-course) MICRO 501 PRINCIPLES OF MICROBIOLOGY 3+1 Objective To teach the students about basics in development of microbiology, differences in prokaryotes and eukaryotic cell and classification of prokaryotes. -
A Rooted Phylogeny Resolves Early Bacterial Evolution (Supplementary Information)
A rooted phylogeny resolves early bacterial evolution (Supplementary Information) All of the files referred to below are provided in the data supplement (extended data files) to our paper, available in the FigShare repository at DOI 10.6084/m9.figshare.12651074. Supplementary Data Extended Data Figures a Fusobacteriota b DST Fusobacteriota DST Cyanobacteria + Cyanobacteria + Margulisbacteria Margulisbacteria Armatimonadota + Armatimonadota + Eremiobacterota Eremiobacterota Terrabacteria Firmicutes/Actinobacteriota Chloroflexota+Dromibacterota/ CPR ACD ACD Armatimonadota/ Eremiobacterota Cyanobacteria/ Margulisbacteria Fusobacteriota DST 0.2 Gracilicutes Gracilicutes Terrabacteria Gracilicutes 0.2 Terrabacteria Spirochaetota Elusimicrobiota c d FCB/PVC Fusobacteriota ACD Fusobacteriota DST Armatimonadota + Eremiobacterota DST Acidobacteriota Cyanobacteria + Margulisbacteria Cyanobacteria + "Proteobacteria"/ Margulisbacteria Armatimonadota + Nitrospirota Eremiobacterota ACD >95% bootstrap support ACD ACD 90-95% bootstrap support <90% bootstrap support 0.2 0.2 Gracilicutes Terrabacteria Gracilicutes Terrabacteria Extended Data Figure 1: Maximum likelihood unrooted bacterial phylogeny under the best-fitting substitution model (LG+C60+R8+F) following removal of the 20%-80% most compositionally heterogeneous sites. Sites were identified and removed using Alignment Pruner. (a) 20% most compositionally heterogeneous removed, with 14580/18234 sites remaining following site stripping; (b) 40% most compositionally heterogeneous removed, with 10941/18234 -

The Neomuran Revolution and Phagotrophic Origin of Eukaryotes and Cilia in the Light of Intracellular Coevolution and a Revised Tree of Life
Downloaded from http://cshperspectives.cshlp.org/ on October 4, 2021 - Published by Cold Spring Harbor Laboratory Press The Neomuran Revolution and Phagotrophic Origin of Eukaryotes and Cilia in the Light of Intracellular Coevolution and a Revised Tree of Life Thomas Cavalier-Smith Department of Zoology, University of Oxford, Oxford OX1 3PS, United Kingdom Correspondence: [email protected] Three kinds of cells exist with increasingly complex membrane-protein targeting: Uni- bacteria (Archaebacteria, Posibacteria) with one cytoplasmic membrane (CM); Negibacte- ria with a two-membrane envelope (inner CM; outer membrane [OM]); eukaryotes with a plasma membrane and topologically distinct endomembranes and peroxisomes. I combine evidence from multigene trees, palaeontology, and cell biology to show that eukaryotes and archaebacteria are sisters, forming the clade neomura that evolved 1.2 Gy ago from a posibacterium, whose DNA segregation and cell division were destabilized by murein wall loss and rescued by the evolving novel neomuran endoskeleton, histones, cytokinesis, and glycoproteins. Phagotrophy then induced coevolving serial major changes making eu- karyote cells, culminating in two dissimilar cilia via a novel gliding–fishing–swimming scenario. I transfer Chloroflexi to Posibacteria, root the universal tree between them and Heliobacteria, and argue that Negibacteria are a clade whose OM, evolving in a green posibacterium, was never lost. THE FIVE KINDS OF CELLS mechanisms of the radical transitions between them. Figure 1 highlights three fundamentally he eukaryotic cell originated by the most different kinds of prokaryote differing great- Tcomplex set of evolutionary changes since ly in membrane topology and membrane and life began: eukaryogenesis. Their complexity wall chemistry. -

Faculty of Agriculture Sciences and Allied Industries
Fundamentals of Plant Pathology FACULTY OF AGRICULTURE SCIENCES AND ALLIED INDUSTRIES Fundamentals of Plant Pathology Course Material Course Name: Fundamentals of Plant Pathology Course Code: PPA-121 B.Sc. Agriculture Semester- II Course Instructor Dr. Kartikay Bisen Faculty of Agricultural Sciences and Allied Industries Rama University, Kanpur Fundamentals of Plant Pathology LECTURE 8 CLASSIFICATION OF BACTERIAL PLANT PATHOGENS CLASSIFICATION Traditionally bacteria have been included in Plantae kingdom under Thallophyta; however, Haeckel in 1966 proposed the kingdom Protista to include all unicellular organisms and placed various organisms of Thallophyta plants and Protozoa animals in Protista. Later, the nucleus character was given more importance. Chatton proposed the most appropriate conceptual basis for taxa at the highest level by recognizing two general patterns of cellular organelles as prokaryotes and eukaryotes in 1937. Stanier (1969) considered prokaryotes as lower protists including blue green algae, myxobacteria and eubacteria; and eukaryotes as higher protists including algae, fungi and protozoa. Prokaryotae was recognised a separate kingdom. However, the correct concept is that of 5 kingdoms according to Whittaker (1969) including Plantae, Animalia, Fungi, Protista and Monera (Prokaryotes). In Bergey‘s Manual of Determinative Bacteriology‘ the phytopathogenic bacteria have been classified into three divisions: Division I – Gracilicutes They include prokaryotes with thin cell walls consisting of outer membrane with fatty acid glycerol ester-type lipids and are usually gram negative. They do not form endospores. Division II – Firmicutes It included prokaryotes with thick (firm) cell wall consisting of peptidoglycan and unit membrane but without any outer membrane. Some of them produce endospore. They are gram positive. Division III – Tenericutes They lack cell wall and cells are enclosed by a unit membrane only.