49 Subpart E—Anticaking Agents Subpart F—Flavoring Agents And
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Effects of Simultaneous Co-Fermentation of Five Indigenous Non-Saccharomyces Strains with S
foods Article Effects of Simultaneous Co-Fermentation of Five Indigenous Non-Saccharomyces Strains with S. cerevisiae on Vidal Icewine Aroma Quality Qian Ge 1,2,3, Chunfeng Guo 1,3, Jing Zhang 2, Yue Yan 2, Danqing Zhao 2, Caihong Li 2, Xiangyu Sun 1, Tingting Ma 1, Tianli Yue 1,3,4 and Yahong Yuan 1,3,4,* 1 College of Food Science and Engineering, Northwest A&F University, Yangling 712100, China; [email protected] (Q.G.); [email protected] (C.G.); [email protected] (X.S.); [email protected] (T.M.); [email protected] (T.Y.) 2 Institute of Quality Standard and Testing Technology for Agro-Products of Ningxia, Yinchuan 750002, China; [email protected] (J.Z.); [email protected] (Y.Y.); [email protected] (D.Z.); [email protected] (C.L.) 3 National Engineering Research Center of Agriculture Integration Test (Yangling), Yangling 712100, China 4 College of Food Science and Technology, Northwest University, Xi’an 710069, China * Correspondence: [email protected]; Tel./Fax: +86-029-87092261 Abstract: In this study, Vidal grape must was fermented using commercial Saccharomyces cerevisiae F33 in pure culture as a control and in mixed culture with five indigenous non-Saccharomyces yeast strains (Hanseniaspora uvarum QTX22, Saccharomycopsis crataegensis YC30, Pichia kluyveri HSP14, Metschnikowia pulcherrima YC12, and Rhodosporidiobolus lusitaniae QTX15) through simultaneous fermentation in a 1:1 ratio. Simultaneous fermentation inhibited the growth of S. cerevisiae F33 and Citation: Ge, Q.; Guo, C.; Zhang, J.; delayed the time to reach the maximum biomass. Compared with pure fermentation, the contents Yan, Y.; Zhao, D.; Li, C.; Sun, X.; Ma, of polyphenols, acetic esters, ethyl esters, other esters, and terpenes were increased by R. -

UPDATED 18Th February 2013
7th February 2015 Welcome to my new seed trade list for 2014-15. 12, 13 and 14 in brackets indicates the harvesting year for the seed. Concerning seed quantity: as I don't have many plants of each species, seed quantity is limited in most cases. Therefore, for some species you may only get a few seeds. Many species are harvested in my garden. Others are surplus from trade and purchase. OUT: Means out of stock. Sometimes I sell surplus seed (if time allows), although this is unlikely this season. NB! Cultivars do not always come true. I offer them anyway, but no guarantees to what you will get! Botanical Name (year of harvest) NB! Traditional vegetables are at the end of the list with (mostly) common English names first. Acanthopanax henryi (14) Achillea sibirica (13) Aconitum lamarckii (12) Achyranthes aspera (14, 13) Adenophora khasiana (13) Adenophora triphylla (13) Agastache anisata (14,13)N Agastache anisata alba (13)N Agastache rugosa (Ex-Japan) (13) (two varieties) Agrostemma githago (13)1 Alcea rosea “Nigra” (13) Allium albidum (13) Allium altissimum (Persian Shallot) (14) Allium atroviolaceum (13) Allium beesianum (14,12) Allium brevistylum (14) Allium caeruleum (14)E Allium carinatum ssp. pulchellum (14) Allium carinatum ssp. pulchellum album (14)E Allium carolinianum (13)N Allium cernuum mix (14) E/N Allium cernuum “Dark Scape” (14)E Allium cernuum ‘Dwarf White” (14)E Allium cernuum ‘Pink Giant’ (14)N Allium cernuum x stellatum (14)E (received as cernuum , but it looks like a hybrid with stellatum, from SSE, OR KA A) Allium cernuum x stellatum (14)E (received as cernuum from a local garden centre) Allium clathratum (13) Allium crenulatum (13) Wild coll. -

Commission Implementing Regulation (EU)
21.3.2013 EN Official Journal of the European Union L 80/1 II (Non-legislative acts) REGULATIONS COMMISSION IMPLEMENTING REGULATION (EU) No 230/2013 of 14 March 2013 on the withdrawal from the market of certain feed additives belonging to the group of flavouring and appetising substances (Text with EEA relevance) THE EUROPEAN COMMISSION, submitted before that deadline for the only animal category for which those feed additives had been auth orised pursuant to Directive 70/524/EEC. Having regard to the Treaty on the Functioning of the European Union, (4) For transparency purposes, feed additives for which no applications for authorisation were submitted within the Having regard to Regulation (EC) No 1831/2003 of the period specified in Article 10(2) of Regulation (EC) No European Parliament and of the Council of 22 September 1831/2003 were listed in a separate part of the 2003 on additives for use in animal nutrition ( 1 ), and in Community Register of Feed Additives. particular Article 10(5) thereof, (5) Those feed additives should therefore be withdrawn from Whereas: the market as far as their use as flavouring and appetising substances is concerned, except for animal species and categories of animal species for which applications for (1) Regulation (EC) No 1831/2003 provides for the auth authorisation have been submitted. This measure does orisation of additives for use in animal nutrition and not interfere with the use of some of the abovemen for the grounds and procedures for granting such auth tioned additives according to other animal species or orisation. Article 10 of that Regulation provides for the categories of animal species or to other functional re-evaluation of additives authorised pursuant to Council groups for which they may be allowed. -

Test Items for Licensing Examination Krok 1 PHARMACY
MINISTRY OF PUBLIC HEALTH OF UKRAINE Department of human resources policy, education and science Testing Board Student ID Last name Variant ________________ Test items for licensing examination Krok 1 PHARMACY (російськомовний варіант) General Instruction Every one of these numbered questions or unfinished statements in this chapter corresponds to answers or statements endings. Choose the answer (finished statements) that fits best and fill in the circle with the corresponding Latin letter on the answer sheet. Authors of items: Abramov A.V., Aleksandrova K.V., Andronov D.Yu., Bilyk O.V., Blinder O.O., Bobyr V.V., Bobrovska O.A., Bohatyriova O.V., Bodnarchuk O.V., Boieva S.S., Bolokhovska T.O., Bondarenko Yu.I., Bratenko M.K., Buchko O.V., Cherneha H.V., Davydova N.V., Deriuhina L.I., Didenko N.O., Dmytriv A.M., Doroshkevych I.O., Dutka N.M., Dynnyk K.V., Filipova L.O., Havryliuk I.M., Herhel T.M., Hlushkova O.M., Hozhdzinsky S.M., Hrekova T.A., Hrechana O.V., Hruzevsky O.A., Hudyvok Ya.S., Hurmak I.S., Ivanets L.M., Ivanov Ye.I., Kartashova T.V., Kava T.V., Kazakova V.V., Kazmirchuk H.V., Kernychna I.Z., Khlus K.M., Khmelnykova L.I., Klebansky Ye.O., Klopotsky H.A., Klymniuk S.I., Kobylinska L.I., Koldunov V.V., Kolesnyk V.P., Kolesnikova T.O., Komlevoy O.M., Kononenko N.M., Kornijevsky Yu.Y., Kremenska L.V., Krushynska T.Yu., Kryzhanovska A.V., Kryshtal M.V., Kukurychkin Ye.R., Kuznietsova N.L., Kuzmina A.V., Lisnycha A.M., Lychko V.H., Makats Ye.F., Maly V.V., Matvijenko A.H., Menchuk K.M., Minarchenko V.M., Mikheiev A.O., Mishchenko -

Seedling Establishment, Bud Movement, and Subterranean Diversity of Geophilous Systems in Apiaceae
Flora (2002) 197, 385–393 http://www.urbanfischer.de/journals/flora Seedling establishment, bud movement, and subterranean diversity of geophilous systems in Apiaceae Norbert Pütz1* & Ina Sukkau2 1 Institute of Nature Conservation and Environmental Education, University of Vechta, Driverstr. 22, D-49377 Vechta, Germany 2 Institute of Botany, RWTH Aachen, Germany * author for correspondence: e-mail: [email protected] Received: Nov 29, 2001 · Accepted: Jun 10, 2002 Summary Geophilous systems of plants are not only regarded as organs of underground storage. Such systems also undergo a large range of modifications in order to fulfill other ‚cryptical‘ functions, e.g. positioning of innovation buds, vegetative cloning, and vege- tative dispersal. Seedlings should always be the point of departure for any investigation into the structure of geophilous systems. This is because in the ability to survive of geophilous plants it is of primary importance that innovation buds can reach a safe position in the soil by the time the first period hostile to vegetation commences. Our analysis of such systems thus focused on examining the development of 34 species of the Apiaceae, beginning with their germination. Independent of life-form and life-span, all species exhibit noticeable terminal bud movement with the aid of contractile organs. Movement was found to be at least 5 mm, reaching a maximum of 45 mm. All species exhibit a noticeable contraction of the primary root. In most cases the contraction phenomenon also occurs in the hypocotyl, and some species show contraction of their lateral and / or adventitious roots. Analysis of movement shows the functional importance of pulling the inno- vation buds down into the soil. -
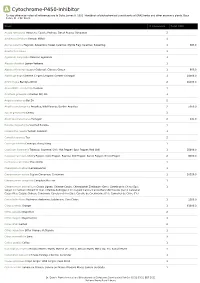
Show Activity
A Cytochrome-P450-Inhibitor *Unless otherwise noted all references are to Duke, James A. 1992. Handbook of phytochemical constituents of GRAS herbs and other economic plants. Boca Raton, FL. CRC Press. Plant # Chemicals Total PPM Acacia farnesiana Huisache; Cassie; Popinac; Sweet Acacia; Opopanax 2 Achillea millefolium Yarrow; Milfoil 1 Acorus calamus Flagroot; Sweetroot; Sweet Calamus; Myrtle Flag; Calamus; Sweetflag 1 384.0 Agastache rugosa 1 Ageratum conyzoides Mexican ageratum 1 Aloysia citrodora Lemon Verbena 1 Alpinia officinarum Lesser Galangal; Chinese Ginger 1 800.0 Alpinia galanga Siamese Ginger; Languas; Greater Galangal 1 24000.0 Ammi majus Bishop's Weed 2 16000.0 Anacardium occidentale Cashew 1 Anethum graveolens Garden Dill; Dill 1 Angelica dahurica Bai Zhi 2 Angelica archangelica Angelica; Wild Parsnip; Garden Angelica 2 5050.0 Apium graveolens Celery 3 Artemisia dracunculus Tarragon 2 141.0 Boronia megastigma Scented Boronia 1 Calamintha nepeta Turkish Calamint 1 Camellia sinensis Tea 2 Cananga odorata Cananga; Ylang-Ylang 1 Capsicum frutescens Tabasco; Cayenne; Chili; Hot Pepper; Spur Pepper; Red Chili 1 35800.0 Capsicum annuum Cherry Pepper; Cone Pepper; Paprika; Bell Pepper; Sweet Pepper; Green Pepper 2 8000.0 Centaurea calcitrapa Star-Thistle 1 Chenopodium album Lambsquarter 1 Cinnamomum verum Ceylon Cinnamon; Cinnamon 1 20320.0 Cinnamomum camphora Camphor; Ho Leaf 1 Cinnamomum aromaticum Cassia Lignea; Chinese Cassia; Chinesischer Zimtbaum (Ger.); Canela de la China (Sp.); 1 Saigon Cinnamon; Chinazimt (Ger.); Kashia-Keihi -

Peucedanum Ostruthium Inhibits E-Selectin and VCAM-1 Expression in Endothelial Cells Through Interference with NF-Κb Signaling
biomolecules Article Peucedanum ostruthium Inhibits E-Selectin and VCAM-1 Expression in Endothelial Cells through Interference with NF-κB Signaling Christoph Lammel 1, Julia Zwirchmayr 2 , Jaqueline Seigner 1 , Judith M. Rollinger 2,* and Rainer de Martin 1 1 Department of Vascular Biology and Thrombosis Research, Medical University of Vienna, Schwarzspanierstaße 17, 1090 Vienna, Austria; [email protected] (C.L.); [email protected] (J.S.); [email protected] (R.d.M.) 2 Department of Pharmacognosy, Faculty of Life Sciences, University of Vienna, Althanstraße 14, 1090 Vienna, Austria; [email protected] * Correspondence: [email protected]; Tel.: +43-1-4277-55255; Fax: +43-1-4277-855255 Received: 5 June 2020; Accepted: 18 August 2020; Published: 21 August 2020 Abstract: Twenty natural remedies traditionally used against different inflammatory diseases were probed for their potential to suppress the expression of the inflammatory markers E-selectin and VCAM-1 in a model system of IL-1 stimulated human umbilical vein endothelial cells (HUVEC). One third of the tested extracts showed in vitro inhibitory effects comparable to the positive control oxozeaenol, an inhibitor of TAK1. Among them, the extract derived from the roots and rhizomes of Peucedanum ostruthium (i.e., Radix Imperatoriae), also known as masterwort, showed a pronounced and dose-dependent inhibitory effect. Reporter gene analysis demonstrated that inhibition takes place on the transcriptional level and involves the transcription factor NF-κB. A more detailed analysis revealed that the P. ostruthium extract (PO) affected the phosphorylation, degradation, and resynthesis of IκBα, the activation of IKKs, and the nuclear translocation of the NF-κB subunit RelA. -

56 Subpart F—Flavoring Agents and Related Substances
§ 172.510 21 CFR Ch. I (4–1–12 Edition) needed to produce its intended effect (a) They are used in the minimum but not in excess of 13 parts per million quantity required to produce their in- calculated as anhydrous sodium ferro- tended physical or technical effect and cyanide. in accordance with all the principles of [42 FR 14491, Mar. 15, 1977, as amended at 58 good manufacturing practice. FR 17098, Apr. 1, 1993] (b) In the appropriate forms (plant parts, fluid and solid extracts, con- Subpart F—Flavoring Agents and centrates, absolutes, oils, gums, bal- Related Substances sams, resins, oleoresins, waxes, and dis- tillates) they consist of one or more of § 172.510 Natural flavoring substances the following, used alone or in com- and natural substances used in con- bination with flavoring substances and junction with flavors. adjuvants generally recognized as safe Natural flavoring substances and in food, previously sanctioned for such natural adjuvants may be safely used use, or regulated in any section of this in food in accordance with the fol- part. lowing conditions. Common name Scientific name Limitations Aloe ................................................................ Aloe perryi Baker, A. barbadensis Mill., A. ferox Mill., and hybrids of this sp. with A. africana Mill. and A. spicata Baker. Althea root and flowers .................................. Althea officinalis L. Amyris (West Indian sandalwood) ................. Amyris balsamifera L. Angola weed .................................................. Roccella fuciformis -

RIFM Fragrance Ingredient Safety Assessment, Menthyl Isovalerate CAS Registry Number 16409-46-4
Food and Chemical Toxicology 110 (2017) S486eS495 Contents lists available at ScienceDirect Food and Chemical Toxicology journal homepage: www.elsevier.com/locate/foodchemtox Short review RIFM fragrance ingredient safety assessment, menthyl isovalerate CAS Registry Number 16409-46-4 * A.M. Api a, , D. Belsito b, D. Botelho a, D. Browne a, M. Bruze c, G.A. Burton Jr. d, J. Buschmann e, M.L. Dagli f, M. Date a, W. Dekant g, C. Deodhar a, M. Francis a, A.D. Fryer h, K. Joshi a,S.LaCavaa, A. Lapczynski a, D.C. Liebler i, D. O'Brien a, R. Parakhia a,A.Patela, T.M. Penning j, G. Ritacco a, J. Romine a, D. Salvito a, T.W. Schultz k, I.G. Sipes l, Y. Thakkar a, E.H. Theophilus a, A.K. Tiethof a, Y. Tokura m, S. Tsang a, J. Wahler a a Research Institute for Fragrance Materials, Inc., 50 Tice Boulevard, Woodcliff Lake, NJ 07677, USA b Member RIFM Expert Panel, Columbia University Medical Center, Department of Dermatology, 161 Fort Washington Ave., New York, NY 10032, USA c Member RIFM Expert Panel, Malmo University Hospital, Department of Occupational & Environmental Dermatology, Sodra Forstadsgatan 101, Entrance 47, Malmo SE-20502, Sweden d Member RIFM Expert Panel, School of Natural Resources & Environment, University of Michigan, Dana Building G110, 440 Church St., Ann Arbor, MI 58109, USA e Member RIFM Expert Panel, Fraunhofer Institute for Toxicology and Experimental Medicine, Nikolai-Fuchs-Strasse 1, 30625 Hannover, Germany f Member RIFM Expert Panel, University of Sao Paulo, School of Veterinary Medicine and Animal Science, Department of Pathology, Av. -

Floristic Quality Assessment Report
FLORISTIC QUALITY ASSESSMENT IN INDIANA: THE CONCEPT, USE, AND DEVELOPMENT OF COEFFICIENTS OF CONSERVATISM Tulip poplar (Liriodendron tulipifera) the State tree of Indiana June 2004 Final Report for ARN A305-4-53 EPA Wetland Program Development Grant CD975586-01 Prepared by: Paul E. Rothrock, Ph.D. Taylor University Upland, IN 46989-1001 Introduction Since the early nineteenth century the Indiana landscape has undergone a massive transformation (Jackson 1997). In the pre-settlement period, Indiana was an almost unbroken blanket of forests, prairies, and wetlands. Much of the land was cleared, plowed, or drained for lumber, the raising of crops, and a range of urban and industrial activities. Indiana’s native biota is now restricted to relatively small and often isolated tracts across the State. This fragmentation and reduction of the State’s biological diversity has challenged Hoosiers to look carefully at how to monitor further changes within our remnant natural communities and how to effectively conserve and even restore many of these valuable places within our State. To meet this monitoring, conservation, and restoration challenge, one needs to develop a variety of appropriate analytical tools. Ideally these techniques should be simple to learn and apply, give consistent results between different observers, and be repeatable. Floristic Assessment, which includes metrics such as the Floristic Quality Index (FQI) and Mean C values, has gained wide acceptance among environmental scientists and decision-makers, land stewards, and restoration ecologists in Indiana’s neighboring states and regions: Illinois (Taft et al. 1997), Michigan (Herman et al. 1996), Missouri (Ladd 1996), and Wisconsin (Bernthal 2003) as well as northern Ohio (Andreas 1993) and southern Ontario (Oldham et al. -

Dr. Duke's Phytochemical and Ethnobotanical Databases List of Plants for Lyme Disease (Chronic)
Dr. Duke's Phytochemical and Ethnobotanical Databases List of Plants for Lyme Disease (Chronic) Plant Chemical Count Activity Count Garcinia xanthochymus 1 1 Nicotiana rustica 1 1 Acacia modesta 1 1 Galanthus nivalis 1 1 Dryopteris marginalis 2 1 Premna integrifolia 1 1 Senecio alpinus 1 1 Cephalotaxus harringtonii 1 1 Comptonia peregrina 1 1 Diospyros rotundifolia 1 1 Alnus crispa 1 1 Haplophyton cimicidum 1 1 Diospyros undulata 1 1 Roylea elegans 1 1 Bruguiera gymnorrhiza 1 1 Gmelina arborea 1 1 Orthosphenia mexicana 1 1 Lumnitzera racemosa 1 1 Melilotus alba 2 1 Duboisia leichhardtii 1 1 Erythroxylum zambesiacum 1 1 Salvia beckeri 1 1 Cephalotaxus spp 1 1 Taxus cuspidata 3 1 Suaeda maritima 1 1 Rhizophora mucronata 1 1 Streblus asper 1 1 Plant Chemical Count Activity Count Dianthus sp. 1 1 Glechoma hirsuta 1 1 Phyllanthus flexuosus 1 1 Euphorbia broteri 1 1 Hyssopus ferganensis 1 1 Lemaireocereus thurberi 1 1 Holacantha emoryi 1 1 Casearia arborea 1 1 Fagonia cretica 1 1 Cephalotaxus wilsoniana 1 1 Hydnocarpus anthelminticus 2 1 Taxus sp 2 1 Zataria multiflora 1 1 Acinos thymoides 1 1 Ambrosia artemisiifolia 1 1 Rhododendron schotense 1 1 Sweetia panamensis 1 1 Thymelaea hirsuta 1 1 Argyreia nervosa 1 1 Carapa guianensis 1 1 Parthenium hysterophorus 1 1 Rhododendron anthopogon 1 1 Strobilanthes cusia 1 1 Dianthus superbus 1 1 Pyropolyporus fomentarius 1 1 Euphorbia hermentiana 1 1 Porteresia coarctata 1 1 2 Plant Chemical Count Activity Count Aerva lanata 1 1 Rivea corymbosa 1 1 Solanum mammosum 1 1 Juniperus horizontalis 1 1 Maytenus -

Volatile Compounds and the Changes in Their Concentration Levelstable
JFS C: Food Chemistry Volatile Compounds and the Changes in Their Concentration Levels during Storage in Beers Containing Varying Malt Concentrations H. TSUJI AND A. MIZUNO C: Food Chemistry ABSTRACT: Volatile compounds in beers brewed with different amounts of malt were analyzed by using the stir bar sorptive extraction–gas chromatography-mass spectrometry method. We identified 90 compounds—25 esters, 17 terpenes, 14 alcohols, 11 acids, 6 furans, 6 aroma compounds, 5 carbonyls, and other compounds. An analysis of aged beer suggested that the concentration levels of stale flavor compounds—β-damascenone, γ -nonalactone, ethyl cinnamate, and 2-methoxy-4-vinylphenol—in nonmalt beer were different from those in all-malt and standard beer. Additionally, concentrations of these compounds did not increase during storage in most nonmalt beer analyzed in this study. Nerolidol may be a good marker candidate regardless of the malt content. Keywords: beer, malt, SBSE, stale flavor, volatile compounds Introduction concentration. SPME has very good reproducibility but limited sen- eer containing a small amount of malt is produced in Japan, sitivity, because the amount of sorptive material that can be coated B primarily because the Japanese government imposes taxes on on the fibers is limited. Stir bar sorptive extraction (SBSE), on the beer based on malt content. Based on malt content, beer and other hand, allows us to detect compounds that are present in very beer-like alcohol beverages in Japan are mainly categorized into 4 small amounts. SBSE has been applied to the analysis of fatty acids types—all-malt beer (100%), standard beer (>67%), low-malt beer (Horak´ and others 2007), terpenoids (Kishimoto and others 2006), (<25%), and nonmalt beer (0%).