Grant Pericyte Continuum 2 AYS 03-01
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-
Cardiovascular System 9
Chapter Cardiovascular System 9 Learning Outcomes On completion of this chapter, you will be able to: 1. State the description and primary functions of the organs/structures of the car- diovascular system. 2. Explain the circulation of blood through the chambers of the heart. 3. Identify and locate the commonly used sites for taking a pulse. 4. Explain blood pressure. 5. Recognize terminology included in the ICD-10-CM. 6. Analyze, build, spell, and pronounce medical words. 7. Comprehend the drugs highlighted in this chapter. 8. Describe diagnostic and laboratory tests related to the cardiovascular system. 9. Identify and define selected abbreviations. 10. Apply your acquired knowledge of medical terms by successfully completing the Practical Application exercise. 255 Anatomy and Physiology The cardiovascular (CV) system, also called the circulatory system, circulates blood to all parts of the body by the action of the heart. This process provides the body’s cells with oxygen and nutritive ele- ments and removes waste materials and carbon dioxide. The heart, a muscular pump, is the central organ of the system. It beats approximately 100,000 times each day, pumping roughly 8,000 liters of blood, enough to fill about 8,500 quart-sized milk cartons. Arteries, veins, and capillaries comprise the network of vessels that transport blood (fluid consisting of blood cells and plasma) throughout the body. Blood flows through the heart, to the lungs, back to the heart, and on to the various body parts. Table 9.1 provides an at-a-glance look at the cardiovascular system. Figure 9.1 shows a schematic overview of the cardiovascular system. -

Vocabulario De Morfoloxía, Anatomía E Citoloxía Veterinaria
Vocabulario de Morfoloxía, anatomía e citoloxía veterinaria (galego-español-inglés) Servizo de Normalización Lingüística Universidade de Santiago de Compostela COLECCIÓN VOCABULARIOS TEMÁTICOS N.º 4 SERVIZO DE NORMALIZACIÓN LINGÜÍSTICA Vocabulario de Morfoloxía, anatomía e citoloxía veterinaria (galego-español-inglés) 2008 UNIVERSIDADE DE SANTIAGO DE COMPOSTELA VOCABULARIO de morfoloxía, anatomía e citoloxía veterinaria : (galego-español- inglés) / coordinador Xusto A. Rodríguez Río, Servizo de Normalización Lingüística ; autores Matilde Lombardero Fernández ... [et al.]. – Santiago de Compostela : Universidade de Santiago de Compostela, Servizo de Publicacións e Intercambio Científico, 2008. – 369 p. ; 21 cm. – (Vocabularios temáticos ; 4). - D.L. C 2458-2008. – ISBN 978-84-9887-018-3 1.Medicina �������������������������������������������������������������������������veterinaria-Diccionarios�������������������������������������������������. 2.Galego (Lingua)-Glosarios, vocabularios, etc. políglotas. I.Lombardero Fernández, Matilde. II.Rodríguez Rio, Xusto A. coord. III. Universidade de Santiago de Compostela. Servizo de Normalización Lingüística, coord. IV.Universidade de Santiago de Compostela. Servizo de Publicacións e Intercambio Científico, ed. V.Serie. 591.4(038)=699=60=20 Coordinador Xusto A. Rodríguez Río (Área de Terminoloxía. Servizo de Normalización Lingüística. Universidade de Santiago de Compostela) Autoras/res Matilde Lombardero Fernández (doutora en Veterinaria e profesora do Departamento de Anatomía e Produción Animal. -

Blood and Lymph Vascular Systems
BLOOD AND LYMPH VASCULAR SYSTEMS BLOOD TRANSFUSIONS Objectives Functions of vessels Layers in vascular walls Classification of vessels Components of vascular walls Control of blood flow in microvasculature Variation in microvasculature Blood barriers Lymphatic system Introduction Multicellular Organisms Need 3 Mechanisms --------------------------------------------------------------- 1. Distribute oxygen, nutrients, and hormones CARDIOVASCULAR SYSTEM 2. Collect waste 3. Transport waste to excretory organs CARDIOVASCULAR SYSTEM Cardiovascular System Component function Heart - Produce blood pressure (systole) Elastic arteries - Conduct blood and maintain pressure during diastole Muscular arteries - Distribute blood, maintain pressure Arterioles - Peripheral resistance and distribute blood Capillaries - Exchange nutrients and waste Venules - Collect blood from capillaries (Edema) Veins - Transmit blood to large veins Reservoir Larger veins - receive lymph and return blood to Heart, blood reservoir Cardiovascular System Heart produces blood pressure (systole) ARTERIOLES – PERIPHERAL RESISTANCE Vessels are structurally adapted to physical and metabolic requirements. Vessels are structurally adapted to physical and metabolic requirements. Cardiovascular System Elastic arteries- conduct blood and maintain pressure during diastole Cardiovascular System Muscular Arteries - distribute blood, maintain pressure Arterioles - peripheral resistance and distribute blood Capillaries - exchange nutrients and waste Venules - collect blood from capillaries -
Why Is Plasma Renin Activity Lower in Populations of African Origin?
Journal of Human Hypertension (2001) 15, 17–25 2001 Macmillan Publishers Ltd All rights reserved 0950-9240/01 $15.00 www.nature.com/jhh REVIEW ARTICLE Why is plasma renin activity lower in populations of African origin? GA Sagnella Blood Pressure Unit, St George’s Hospital Medical School, Cranmer Terrace, London SW17 ORE, UK Plasma renin activity is significantly lower in black the molecular level suggests that the lower PRA may people compared with whites independent of age and arise from gene variation in the renal epithelial sodium blood pressure status. The lower PRA appears to be due channel. The functional significance of the lower PRA in to a reduction in the rate of secretion of renin but the relation to the different pattern of cardiovascular and exact mechanistic events underlying such differences in renal disease between blacks and whites remains renin release between blacks and whites are still not unclear. Moreover, direct investigations of pre-treat- fully understood. Nevertheless, given the paramount ment renin status in hypertensive blacks in relation to importance of the renin-angiotensin system in the con- blood pressure response have demonstrated that the trol of sodium balance, a most likely explanation is that pre-treatment PRA is not a good index of subsequent the lower renin is a consequence of differences in renal blood pressure response to pharmacological treatment. sodium handling between blacks and whites. The lower Nevertheless, the blood pressure reduction to short PRA does not reflect differences in dietary sodium term sodium restriction is greater in blacks compared intake but the evidence available suggests that the low with whites and, in the black subjects, the greater PRA could be part of the corrective mechanisms reduction in blood pressure to sodium restriction designed to maintain sodium balance in the presence appears to be related, at least in part, to the decreased of an increased tendency for sodium retention in black responsiveness of the renin-angiotensin system. -
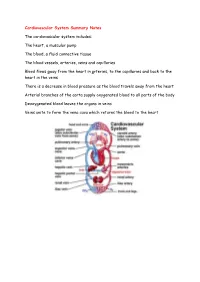
Cardiovascular System Summary Notes the Cardiovascular System
Cardiovascular System Summary Notes The cardiovascular system includes: The heart, a muscular pump The blood, a fluid connective tissue The blood vessels, arteries, veins and capillaries Blood flows away from the heart in arteries, to the capillaries and back to the heart in the veins There is a decrease in blood pressure as the blood travels away from the heart Arterial branches of the aorta supply oxygenated blood to all parts of the body Deoxygenated blood leaves the organs in veins Veins unite to form the vena cava which returns the blood to the heart Pulmonary System This is the route by which blood is circulated from the heart to the lungs and back to the heart again The pulmonary system is exceptional in that the pulmonary artery carries deoxygenated blood and the pulmonary vein carries oxygenated blood Hepatic Portal Vein There is another exception in the circulatory system – the hepatic portal vein Veins normally carry blood from an organ back to the heart The hepatic portal vein carries blood from the capillary bed of the intestine to the capillary bed of the liver As a result, the liver has three blood vessels associated with it Arteries and Veins The central cavity of a blood vessel is called the lumen The lumen is lined with a thin layer of cells called the endothelium The composition of the vessel wall surrounding the endothelium is different in arteries, veins and capillaries Arteries carry blood away from the heart Arteries have a thick middle layer of smooth muscle They have an inner and outer layer of elastic fibres Elastic -

Gli1+ Pericyte Loss Induces Capillary Rarefaction and Proximal Tubular Injury
BRIEF COMMUNICATION www.jasn.org Gli1+ Pericyte Loss Induces Capillary Rarefaction and Proximal Tubular Injury † ‡ ‡ ‡ Rafael Kramann,* Janewit Wongboonsin, § Monica Chang-Panesso, Flavia G. Machado, and ‡ Benjamin D. Humphreys *Renal Division, Brigham and Women’s Hospital, Department of Medicine, Harvard Medical School, Boston, Massachusetts; †Division of Nephrology and Clinical Immunology, RWTH Aachen University Medical Faculty, RWTH Aachen University, Aachen, Germany; ‡Division of Nephrology, Department of Medicine, Washington University School of Medicine in St. Louis, St. Louis, Missouri; and §Department of Medicine, Siriraj Hospital, Mahidol University, Bangkok, Thailand ABSTRACT Peritubular capillary rarefaction is hypothesized to contribute to the increased risk of after AKI, and whether pericyte loss is future CKD after AKI. Here, we directly tested the role of Gli1+ kidney pericytes in the sufficient to induce peritubular capillary maintenance of peritubular capillary health, and the consequences of pericyte loss loss and altered morphology. during injury. Using bigenic Gli1-CreERt2; R26tdTomato reporter mice, we observed We genetically labeled pericytes using increased distance between Gli1+ pericytes and endothelial cells after AKI (mean6 Gli1-CreERt2 mice crossed against SEM: 3.360.1 mm before injury versus 12.560.2 mm after injury; P,0.001). Using a the R26tdTomato reporter mouse (Gt genetic ablation model, we asked whether pericyte loss alone is sufficient for capillary (ROSA)26Sortm9(CAF-tdTomato)Hze/J; destabilization. Ten days after pericyte ablation, we observed endothelial cell damage Figure 1A). After tamoxifen injection, mice by electron microscopy. Furthermore, pericyte loss led to significantly reduced capil- were subjected to severe unilateral ische- lary number at later time points (mean6SEM capillaries/high-power field: 67.664.7 in mia reperfusion injury (IRI; 28-minute control versus 44.164.8 at 56 days; P,0.05) and increased cross-sectional area (mean6 clamp). -

SGLT2 Inhibition and Potential Renal Protection
The knowns and unknowns of SGLT2 inhibition in CKD Paola Fioretto, MD Padua, Italy June 14, 2019 - Budapest, Hungary SGLT2 inhibition in CKD: Discussing the key questions and evidence Budapest, june 14 2019 The knowns and unknowns of SGLT2 inhibition in CKD Paola Fioretto Department of Medicine University of Padova, Italy 180 g of glucose filtered Glomerulus Proximal tubule Distal tubule Collecting duct each day S1 S2 Glucose filtration S3 SGLT2 SGLT1 90% 10% Glucose reabsorption Loop of Henle Up to ~ 90% of glucose ~ 10% of glucose Minimal is reabsorbed is reabsorbed glucose from the S1/S2 segments from the S3 segment excretion Possible mechanisms responsible for cardiovascular and renal protection with SGLT2 inhibition SGLT2 inhibition Glycosuria Natriuresis ↓Blood ↓Plasma Negative caloric balance ↑Uricosuria pressure ↑Tubuloglomerular volume feedback ↓Myocardial ↓HbA1c ↓ Afferent stretch ↓ ↓Plasma uric ↓Arterial ↓ arteriole acid stiffness ↑ constriction ↓Total body fat mass ↓Inflammation ↓Glucose toxicity ↓Epicardial fat ↓Intraglomerular hypertension ↓Ventricular ↓Hyperfiltration arrhythmias ↓Atherosclerosis Activation of ACE2 – Ang1/7 ↑Cardiac contractility ↓Inflammation No sympathetic nervous system activation ↓Fibrosis Cardiac and renal protection Heerspink HJ et al, Circulation 2016 Tonneijck et al, J Am Soc Nephrol 2017 Diabetic nephron Diabetic nephron with SGLT2 i Effects of SGLT2 i on afferent arteriole tone: in vivo studies with multiphoton microscope imaging techniques Kidokoro K et al, Circulation 2019 Effects of SGLT2 i -

The Dynamic Roles of Red Blood Cell in Microcirculation
Rochester Institute of Technology RIT Scholar Works Theses 7-15-2019 The Dynamic Roles of Red Blood Cell in Microcirculation Sitong Zhou [email protected] Follow this and additional works at: https://scholarworks.rit.edu/theses Recommended Citation Zhou, Sitong, "The Dynamic Roles of Red Blood Cell in Microcirculation" (2019). Thesis. Rochester Institute of Technology. Accessed from This Dissertation is brought to you for free and open access by RIT Scholar Works. It has been accepted for inclusion in Theses by an authorized administrator of RIT Scholar Works. For more information, please contact [email protected]. R.I.T The Dynamic Roles of Red Blood Cell in Microcirculation by Sitong Zhou A dissertation submitted in partial fulfillment of the requirements for the degree of Doctorate of Philosophy in Microsystems Engineering Microsystems Engineering Program Kate Gleason College of Engineering Rochester Institute of Technology Rochester, New York July 15th, 2019 1 The Dynamic Roles of Red Blood Cell in Microcirculation by Sitong Zhou Committee Approval: We, the undersigned committee members, certify that we have advised and/or supervised the candidate on the work described in this dissertation. We further certify that we have reviewed the dissertation manuscript and approve it in partial fulfillment of the requirements of the degree of Doctor of Philosophy in Microsystems Engineering. ______________________________________________________________________________ Dr. Jiandi Wan Date Assistant Professor, Chemical Engineering ______________________________________________________________________________ -

Prostaglandin E2 Breaks Down Pericyte–Endothelial Cell Interaction Via EP1 and EP4-Dependent Downregulation of Pericyte N-Cadh
www.nature.com/scientificreports OPEN Prostaglandin E2 breaks down pericyte–endothelial cell interaction via EP1 and EP4‑dependent downregulation of pericyte N‑cadherin, connexin‑43, and R‑Ras Carole Y. Perrot1,2, Jose L. Herrera1,2, Ashley E. Fournier‑Goss1,2 & Masanobu Komatsu1,2* A close association between pericytes and endothelial cells (ECs) is crucial to the stability and function of capillary blood vessels and microvessels. The loss or dysfunction of pericytes results in signifcant disruption of these blood vessels as observed in pathological conditions, including cancer, diabetes, stroke, and Alzheimer’s disease. Prostaglandin E2 (PGE2) is a lipid mediator of infammation, and its tissue concentration is elevated in cancer and neurological disorders. Here, we show that the exposure to PGE2 switches pericytes to a fast‑migrating, loosely adhered phenotype that fails to intimately interact with ECs. N‑cadherin and connexin‑43 in adherens junction and gap junction between pericytes and ECs are downregulated by EP‑4 and EP‑1‑dependent mechanisms, leading to breakdown of the pericyte–EC interaction. Furthermore, R‑Ras, a small GTPase important for vascular normalization and vessel stability, is transcriptionally repressed by PGE2 in an EP4‑dependent manner. Mouse dermal capillary vessels lose pericyte coverage substantially upon PGE2 injection into the skin. Our results suggest that EP‑mediated direct disruption of pericytes by PGE2 is a key process for vascular destabilization. Restoring pericyte–EC interaction using inhibitors of PGE2 signaling may ofer a therapeutic strategy in cancer and neurological disorders, in which pericyte dysfunction contributes to the disease progression. Pericytes are mesenchyme-derived mural cells that surround endothelial cells (ECs) of capillary blood vessels and microvessels. -

Nomina Histologica Veterinaria, First Edition
NOMINA HISTOLOGICA VETERINARIA Submitted by the International Committee on Veterinary Histological Nomenclature (ICVHN) to the World Association of Veterinary Anatomists Published on the website of the World Association of Veterinary Anatomists www.wava-amav.org 2017 CONTENTS Introduction i Principles of term construction in N.H.V. iii Cytologia – Cytology 1 Textus epithelialis – Epithelial tissue 10 Textus connectivus – Connective tissue 13 Sanguis et Lympha – Blood and Lymph 17 Textus muscularis – Muscle tissue 19 Textus nervosus – Nerve tissue 20 Splanchnologia – Viscera 23 Systema digestorium – Digestive system 24 Systema respiratorium – Respiratory system 32 Systema urinarium – Urinary system 35 Organa genitalia masculina – Male genital system 38 Organa genitalia feminina – Female genital system 42 Systema endocrinum – Endocrine system 45 Systema cardiovasculare et lymphaticum [Angiologia] – Cardiovascular and lymphatic system 47 Systema nervosum – Nervous system 52 Receptores sensorii et Organa sensuum – Sensory receptors and Sense organs 58 Integumentum – Integument 64 INTRODUCTION The preparations leading to the publication of the present first edition of the Nomina Histologica Veterinaria has a long history spanning more than 50 years. Under the auspices of the World Association of Veterinary Anatomists (W.A.V.A.), the International Committee on Veterinary Anatomical Nomenclature (I.C.V.A.N.) appointed in Giessen, 1965, a Subcommittee on Histology and Embryology which started a working relation with the Subcommittee on Histology of the former International Anatomical Nomenclature Committee. In Mexico City, 1971, this Subcommittee presented a document entitled Nomina Histologica Veterinaria: A Working Draft as a basis for the continued work of the newly-appointed Subcommittee on Histological Nomenclature. This resulted in the editing of the Nomina Histologica Veterinaria: A Working Draft II (Toulouse, 1974), followed by preparations for publication of a Nomina Histologica Veterinaria. -

Role of Pericytes in the Retina
Eye (2018) 32, 483–486 © 2018 Macmillan Publishers Limited, part of Springer Nature. All rights reserved 0950-222X/18 www.nature.com/eye Role of pericytes in the COMMENT retina Eye (2018) 32, 483–486; doi:10.1038/eye.2017.220; In contrast, Park and colleagues demonstrated published online 10 November 2017 using state-of-the-art techniques, including deletion of several genes from endothelial cells, that PDGFB/PDGFRβ signaling is indispensable Diabetic retinopathy is a major severe ocular in the formation and maturation of blood- complication associated with the metabolic retinal-barrier at the postnatal stage through disorder of diabetes mellitus.1 The lack of a active recruitment of pericytes onto the growing detailed knowledge about the cellular and retinal vessels.3 Additionally, the authors molecular mechanisms involved in diabetic revealed that pericytes are important in the adult retinopathy restricts the design of effective retina as regulators, as they control the treatments. Understanding the roles of retinal expression of several genes (FOXO1, Ang2, and cells during this process is of utmost importance, VEGFR2) to protect retinal vessels against since gaining control of specific cell populations injuries and stresses.3 fi may allow us to arrest or even induce reversion Here, we discuss the ndings from this work, of diabetic retinopathy. and evaluate recent advances in our Pericyte dropout or loss has been suggested to understanding of pericytes roles in the retina. have great consequences on blood vessel fi remodeling, and possibly causes the rst Perspectives/future directions abnormalities of the diabetic eye which can be fi observed clinically in diabetic retinopathy.2 The ndings from this study are based on the expression of PDGFRβ in pericytes. -

Blood Vessels and Circulation
19 Blood Vessels and Circulation Lecture Presentation by Lori Garrett © 2018 Pearson Education, Inc. Section 1: Functional Anatomy of Blood Vessels Learning Outcomes 19.1 Distinguish between the pulmonary and systemic circuits, and identify afferent and efferent blood vessels. 19.2 Distinguish among the types of blood vessels on the basis of their structure and function. 19.3 Describe the structures of capillaries and their functions in the exchange of dissolved materials between blood and interstitial fluid. 19.4 Describe the venous system, and indicate the distribution of blood within the cardiovascular system. © 2018 Pearson Education, Inc. Module 19.1: The heart pumps blood, in sequence, through the arteries, capillaries, and veins of the pulmonary and systemic circuits Blood vessels . Blood vessels conduct blood between the heart and peripheral tissues . Arteries (carry blood away from the heart) • Also called efferent vessels . Veins (carry blood to the heart) • Also called afferent vessels . Capillaries (exchange substances between blood and tissues) • Interconnect smallest arteries and smallest veins © 2018 Pearson Education, Inc. Module 19.1: Blood vessels and circuits Two circuits 1. Pulmonary circuit • To and from gas exchange surfaces in the lungs 2. Systemic circuit • To and from rest of body © 2018 Pearson Education, Inc. Module 19.1: Blood vessels and circuits Circulation pathway through circuits 1. Right atrium (entry chamber) • Collects blood from systemic circuit • To right ventricle to pulmonary circuit 2. Pulmonary circuit • Pulmonary arteries to pulmonary capillaries to pulmonary veins © 2018 Pearson Education, Inc. Module 19.1: Blood vessels and circuits Circulation pathway through circuits (continued) 3. Left atrium • Receives blood from pulmonary circuit • To left ventricle to systemic circuit 4.