Goldfish Tail Circulation Lab
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Chapter 20 *Lecture Powerpoint the Circulatory System: Blood Vessels and Circulation
Chapter 20 *Lecture PowerPoint The Circulatory System: Blood Vessels and Circulation *See separate FlexArt PowerPoint slides for all figures and tables preinserted into PowerPoint without notes. Copyright © The McGraw-Hill Companies, Inc. Permission required for reproduction or display. Introduction • The route taken by the blood after it leaves the heart was a point of much confusion for many centuries – Chinese emperor Huang Ti (2697–2597 BC) believed that blood flowed in a complete circuit around the body and back to the heart – Roman physician Galen (129–c. 199) thought blood flowed back and forth like air; the liver created blood out of nutrients and organs consumed it – English physician William Harvey (1578–1657) did experimentation on circulation in snakes; birth of experimental physiology – After microscope was invented, blood and capillaries were discovered by van Leeuwenhoek and Malpighi 20-2 General Anatomy of the Blood Vessels • Expected Learning Outcomes – Describe the structure of a blood vessel. – Describe the different types of arteries, capillaries, and veins. – Trace the general route usually taken by the blood from the heart and back again. – Describe some variations on this route. 20-3 General Anatomy of the Blood Vessels Copyright © The McGraw-Hill Companies, Inc. Permission required for reproduction or display. Capillaries Artery: Tunica interna Tunica media Tunica externa Nerve Vein Figure 20.1a (a) 1 mm © The McGraw-Hill Companies, Inc./Dennis Strete, photographer • Arteries carry blood away from heart • Veins -
Cardiovascular System 9
Chapter Cardiovascular System 9 Learning Outcomes On completion of this chapter, you will be able to: 1. State the description and primary functions of the organs/structures of the car- diovascular system. 2. Explain the circulation of blood through the chambers of the heart. 3. Identify and locate the commonly used sites for taking a pulse. 4. Explain blood pressure. 5. Recognize terminology included in the ICD-10-CM. 6. Analyze, build, spell, and pronounce medical words. 7. Comprehend the drugs highlighted in this chapter. 8. Describe diagnostic and laboratory tests related to the cardiovascular system. 9. Identify and define selected abbreviations. 10. Apply your acquired knowledge of medical terms by successfully completing the Practical Application exercise. 255 Anatomy and Physiology The cardiovascular (CV) system, also called the circulatory system, circulates blood to all parts of the body by the action of the heart. This process provides the body’s cells with oxygen and nutritive ele- ments and removes waste materials and carbon dioxide. The heart, a muscular pump, is the central organ of the system. It beats approximately 100,000 times each day, pumping roughly 8,000 liters of blood, enough to fill about 8,500 quart-sized milk cartons. Arteries, veins, and capillaries comprise the network of vessels that transport blood (fluid consisting of blood cells and plasma) throughout the body. Blood flows through the heart, to the lungs, back to the heart, and on to the various body parts. Table 9.1 provides an at-a-glance look at the cardiovascular system. Figure 9.1 shows a schematic overview of the cardiovascular system. -

Blood and Lymph Vascular Systems
BLOOD AND LYMPH VASCULAR SYSTEMS BLOOD TRANSFUSIONS Objectives Functions of vessels Layers in vascular walls Classification of vessels Components of vascular walls Control of blood flow in microvasculature Variation in microvasculature Blood barriers Lymphatic system Introduction Multicellular Organisms Need 3 Mechanisms --------------------------------------------------------------- 1. Distribute oxygen, nutrients, and hormones CARDIOVASCULAR SYSTEM 2. Collect waste 3. Transport waste to excretory organs CARDIOVASCULAR SYSTEM Cardiovascular System Component function Heart - Produce blood pressure (systole) Elastic arteries - Conduct blood and maintain pressure during diastole Muscular arteries - Distribute blood, maintain pressure Arterioles - Peripheral resistance and distribute blood Capillaries - Exchange nutrients and waste Venules - Collect blood from capillaries (Edema) Veins - Transmit blood to large veins Reservoir Larger veins - receive lymph and return blood to Heart, blood reservoir Cardiovascular System Heart produces blood pressure (systole) ARTERIOLES – PERIPHERAL RESISTANCE Vessels are structurally adapted to physical and metabolic requirements. Vessels are structurally adapted to physical and metabolic requirements. Cardiovascular System Elastic arteries- conduct blood and maintain pressure during diastole Cardiovascular System Muscular Arteries - distribute blood, maintain pressure Arterioles - peripheral resistance and distribute blood Capillaries - exchange nutrients and waste Venules - collect blood from capillaries -

In Sickness and in Health: the Immunological Roles of the Lymphatic System
International Journal of Molecular Sciences Review In Sickness and in Health: The Immunological Roles of the Lymphatic System Louise A. Johnson MRC Human Immunology Unit, MRC Weatherall Institute of Molecular Medicine, University of Oxford, John Radcliffe Hospital, Headington, Oxford OX3 9DS, UK; [email protected] Abstract: The lymphatic system plays crucial roles in immunity far beyond those of simply providing conduits for leukocytes and antigens in lymph fluid. Endothelial cells within this vasculature are dis- tinct and highly specialized to perform roles based upon their location. Afferent lymphatic capillaries have unique intercellular junctions for efficient uptake of fluid and macromolecules, while expressing chemotactic and adhesion molecules that permit selective trafficking of specific immune cell subsets. Moreover, in response to events within peripheral tissue such as inflammation or infection, soluble factors from lymphatic endothelial cells exert “remote control” to modulate leukocyte migration across high endothelial venules from the blood to lymph nodes draining the tissue. These immune hubs are highly organized and perfectly arrayed to survey antigens from peripheral tissue while optimizing encounters between antigen-presenting cells and cognate lymphocytes. Furthermore, subsets of lymphatic endothelial cells exhibit differences in gene expression relating to specific func- tions and locality within the lymph node, facilitating both innate and acquired immune responses through antigen presentation, lymph node remodeling and regulation of leukocyte entry and exit. This review details the immune cell subsets in afferent and efferent lymph, and explores the mech- anisms by which endothelial cells of the lymphatic system regulate such trafficking, for immune surveillance and tolerance during steady-state conditions, and in response to infection, acute and Citation: Johnson, L.A. -

Arterial System Lecture Block 10 Vascular Structure/Function
Arterial System Lecture Block 10 Arterial System Bioengineering 6000 CV Physiology Vascular Structure/Function Arterial System Bioengineering 6000 CV Physiology Functional Overview Arterial System Bioengineering 6000 CV Physiology Vessel Structure Aorta Artery Vein Vena Cava Arteriole Capillary Venule Diameter 25 mm 4 mm 5 mm 30 mm 30 µm 8 µm 20 µm Wall 2 mm 1 mm 0.5 mm 1.5 mm 6 µm 0.5 µm 1 µm thickness Endothelium Elastic tissue Smooth Muscle Fibrous Tissue Arterial System Bioengineering 6000 CV Physiology Aortic Compliance • Factors: – age 20--24 yrs – athersclerosis 300 • Effects – more pulsatile flow 200 dV 30--40 yrs C = – more cardiac work dP 50-60 yrs – not hypertension Laplace’s Law 100 70--75 yrs (thin-walled cylinder): [%] Volume Blood T = wall tension P = pressure T = Pr r = radius For thick wall cylinder 100 150 200 P = pressure Pr Pressure [mm Hg] σ = wall stress r = radius σ = Tension Wall Stress w = wall thickness w [dyne/cm] [dyne/cm2] Aorta 2 x 105 10 x 105 Capillary 15-70 1.5 x 105 Arterial System Bioengineering 6000 CV Physiology Arterial Hydraulic Filter Arterial System Bioengineering 6000 CV Physiology Arterial System as Hydraulic Filter Arterial Cardiac Pressure • Pulsatile --> Output t Physiological smooth flow t Ideal • Cardiac energy conversion Cardiac • Reduces total Output Arterial cardiac work Pressure t Pulsatile t Challenge Cardiac Output Arterial Pressure t Filtered t Reality Arterial System Bioengineering 6000 CV Physiology Elastic Recoil in Arteries Arterial System Bioengineering 6000 CV Physiology Effects of Vascular Resistance and Compliance Arterial System Bioengineering 6000 CV Physiology Cardiac Output vs. -
Why Is Plasma Renin Activity Lower in Populations of African Origin?
Journal of Human Hypertension (2001) 15, 17–25 2001 Macmillan Publishers Ltd All rights reserved 0950-9240/01 $15.00 www.nature.com/jhh REVIEW ARTICLE Why is plasma renin activity lower in populations of African origin? GA Sagnella Blood Pressure Unit, St George’s Hospital Medical School, Cranmer Terrace, London SW17 ORE, UK Plasma renin activity is significantly lower in black the molecular level suggests that the lower PRA may people compared with whites independent of age and arise from gene variation in the renal epithelial sodium blood pressure status. The lower PRA appears to be due channel. The functional significance of the lower PRA in to a reduction in the rate of secretion of renin but the relation to the different pattern of cardiovascular and exact mechanistic events underlying such differences in renal disease between blacks and whites remains renin release between blacks and whites are still not unclear. Moreover, direct investigations of pre-treat- fully understood. Nevertheless, given the paramount ment renin status in hypertensive blacks in relation to importance of the renin-angiotensin system in the con- blood pressure response have demonstrated that the trol of sodium balance, a most likely explanation is that pre-treatment PRA is not a good index of subsequent the lower renin is a consequence of differences in renal blood pressure response to pharmacological treatment. sodium handling between blacks and whites. The lower Nevertheless, the blood pressure reduction to short PRA does not reflect differences in dietary sodium term sodium restriction is greater in blacks compared intake but the evidence available suggests that the low with whites and, in the black subjects, the greater PRA could be part of the corrective mechanisms reduction in blood pressure to sodium restriction designed to maintain sodium balance in the presence appears to be related, at least in part, to the decreased of an increased tendency for sodium retention in black responsiveness of the renin-angiotensin system. -
Cardiovascular System Summary Notes the Cardiovascular System
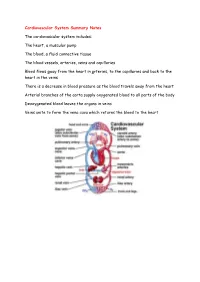
Cardiovascular System Summary Notes The cardiovascular system includes: The heart, a muscular pump The blood, a fluid connective tissue The blood vessels, arteries, veins and capillaries Blood flows away from the heart in arteries, to the capillaries and back to the heart in the veins There is a decrease in blood pressure as the blood travels away from the heart Arterial branches of the aorta supply oxygenated blood to all parts of the body Deoxygenated blood leaves the organs in veins Veins unite to form the vena cava which returns the blood to the heart Pulmonary System This is the route by which blood is circulated from the heart to the lungs and back to the heart again The pulmonary system is exceptional in that the pulmonary artery carries deoxygenated blood and the pulmonary vein carries oxygenated blood Hepatic Portal Vein There is another exception in the circulatory system – the hepatic portal vein Veins normally carry blood from an organ back to the heart The hepatic portal vein carries blood from the capillary bed of the intestine to the capillary bed of the liver As a result, the liver has three blood vessels associated with it Arteries and Veins The central cavity of a blood vessel is called the lumen The lumen is lined with a thin layer of cells called the endothelium The composition of the vessel wall surrounding the endothelium is different in arteries, veins and capillaries Arteries carry blood away from the heart Arteries have a thick middle layer of smooth muscle They have an inner and outer layer of elastic fibres Elastic -

SGLT2 Inhibition and Potential Renal Protection
The knowns and unknowns of SGLT2 inhibition in CKD Paola Fioretto, MD Padua, Italy June 14, 2019 - Budapest, Hungary SGLT2 inhibition in CKD: Discussing the key questions and evidence Budapest, june 14 2019 The knowns and unknowns of SGLT2 inhibition in CKD Paola Fioretto Department of Medicine University of Padova, Italy 180 g of glucose filtered Glomerulus Proximal tubule Distal tubule Collecting duct each day S1 S2 Glucose filtration S3 SGLT2 SGLT1 90% 10% Glucose reabsorption Loop of Henle Up to ~ 90% of glucose ~ 10% of glucose Minimal is reabsorbed is reabsorbed glucose from the S1/S2 segments from the S3 segment excretion Possible mechanisms responsible for cardiovascular and renal protection with SGLT2 inhibition SGLT2 inhibition Glycosuria Natriuresis ↓Blood ↓Plasma Negative caloric balance ↑Uricosuria pressure ↑Tubuloglomerular volume feedback ↓Myocardial ↓HbA1c ↓ Afferent stretch ↓ ↓Plasma uric ↓Arterial ↓ arteriole acid stiffness ↑ constriction ↓Total body fat mass ↓Inflammation ↓Glucose toxicity ↓Epicardial fat ↓Intraglomerular hypertension ↓Ventricular ↓Hyperfiltration arrhythmias ↓Atherosclerosis Activation of ACE2 – Ang1/7 ↑Cardiac contractility ↓Inflammation No sympathetic nervous system activation ↓Fibrosis Cardiac and renal protection Heerspink HJ et al, Circulation 2016 Tonneijck et al, J Am Soc Nephrol 2017 Diabetic nephron Diabetic nephron with SGLT2 i Effects of SGLT2 i on afferent arteriole tone: in vivo studies with multiphoton microscope imaging techniques Kidokoro K et al, Circulation 2019 Effects of SGLT2 i -

Lymph and Lymphatic Vessels
Cardiovascular System LYMPH AND LYMPHATIC VESSELS Venous system Arterial system Large veins Heart (capacitance vessels) Elastic arteries Large (conducting lymphatic vessels) vessels Lymph node Muscular arteries (distributing Lymphatic vessels) system Small veins (capacitance Arteriovenous vessels) anastomosis Lymphatic Sinusoid capillary Arterioles (resistance vessels) Postcapillary Terminal arteriole venule Metarteriole Thoroughfare Capillaries Precapillary sphincter channel (exchange vessels) Copyright © 2010 Pearson Education, Inc. Figure 19.2 Regional Internal jugular vein lymph nodes: Cervical nodes Entrance of right lymphatic duct into vein Entrance of thoracic duct into vein Axillary nodes Thoracic duct Cisterna chyli Aorta Inguinal nodes Lymphatic collecting vessels Drained by the right lymphatic duct Drained by the thoracic duct (a) General distribution of lymphatic collecting vessels and regional lymph nodes. Figure 20.2a Lymphatic System Outflow of fluid slightly exceeds return Consists of three parts 1. A network of lymphatic vessels carrying lymph 1. Transports fluid back to CV system 2. Lymph nodes 1. Filter the fluid within the vessels 3. Lymphoid organs 1. Participate in disease prevention Lymphatic System Functions 1. Returns interstitial fluid and leaked plasma proteins back to the blood 2. Disease surveillance 3. Lipid transport from intestine via lacteals Venous system Arterial system Heart Lymphatic system: Lymph duct Lymph trunk Lymph node Lymphatic collecting vessels, with valves Tissue fluid Blood Lymphatic capillaries Tissue cell capillary Blood Lymphatic capillaries capillaries (a) Structural relationship between a capillary bed of the blood vascular system and lymphatic capillaries. Filaments anchored to connective tissue Endothelial cell Flaplike minivalve Fibroblast in loose connective tissue (b) Lymphatic capillaries are blind-ended tubes in which adjacent endothelial cells overlap each other, forming flaplike minivalves. -

Fluctuating and Sensory-Induced Vasodynamics in Rodent Cortex Extend Arteriole Capacity
Fluctuating and sensory-induced vasodynamics in rodent cortex extend arteriole capacity Patrick J. Drewa,b,c, Andy Y. Shiha, and David Kleinfelda,d,1 aDepartments of Physics and Neurobiology and dCenter for Neural Circuits and Behavior, University of California, San Diego, CA 92093; and bCenter for Neural Engineering and cDepartments of Engineering Science and Mechanics and Neurosurgery, Pennsylvania State University, University Park, PA 16802 Edited* by Nikos K. Logothetis, Max Planck Institute for Biological Cybernetics, Tübingen, Germany, and approved March 28, 2011 (received for review January 10, 2011) Neural activity in the brain is followed by localized changes in blood Fig. 1E). Last, the slow spontaneous oscillations in arterial flow and volume. We address the relative change in volume for diameter seen in awake animals (Fig. 1C and Fig. S1) are greatly arteriole vs. venous blood within primary vibrissa cortex of awake, attenuated by urethane anesthesia (Fig. S2). head-fixed mice. Two-photon laser-scanning microscopy was used to In contrast to the case for arteries, spontaneous changes in the measure spontaneous and sensory evoked changes in flow and diameter of surface venules are relatively small (Fig. 1 B and C volume at the level of single vessels. We find that arterioles exhibit and Fig. S2). The spectral content of these signals is similar to that slow (<1 Hz) spontaneous increases in their diameter, as well as pro- seen for arterioles, although the amplitudes at low frequencies nounced dilation in response to both punctate and prolonged stim- are substantially reduced (Fig. 1 C and D). Further, there was ulation of the contralateral vibrissae. -

Blood Vessels and Circulation
19 Blood Vessels and Circulation Lecture Presentation by Lori Garrett © 2018 Pearson Education, Inc. Section 1: Functional Anatomy of Blood Vessels Learning Outcomes 19.1 Distinguish between the pulmonary and systemic circuits, and identify afferent and efferent blood vessels. 19.2 Distinguish among the types of blood vessels on the basis of their structure and function. 19.3 Describe the structures of capillaries and their functions in the exchange of dissolved materials between blood and interstitial fluid. 19.4 Describe the venous system, and indicate the distribution of blood within the cardiovascular system. © 2018 Pearson Education, Inc. Module 19.1: The heart pumps blood, in sequence, through the arteries, capillaries, and veins of the pulmonary and systemic circuits Blood vessels . Blood vessels conduct blood between the heart and peripheral tissues . Arteries (carry blood away from the heart) • Also called efferent vessels . Veins (carry blood to the heart) • Also called afferent vessels . Capillaries (exchange substances between blood and tissues) • Interconnect smallest arteries and smallest veins © 2018 Pearson Education, Inc. Module 19.1: Blood vessels and circuits Two circuits 1. Pulmonary circuit • To and from gas exchange surfaces in the lungs 2. Systemic circuit • To and from rest of body © 2018 Pearson Education, Inc. Module 19.1: Blood vessels and circuits Circulation pathway through circuits 1. Right atrium (entry chamber) • Collects blood from systemic circuit • To right ventricle to pulmonary circuit 2. Pulmonary circuit • Pulmonary arteries to pulmonary capillaries to pulmonary veins © 2018 Pearson Education, Inc. Module 19.1: Blood vessels and circuits Circulation pathway through circuits (continued) 3. Left atrium • Receives blood from pulmonary circuit • To left ventricle to systemic circuit 4. -

Quantitative Model for Predicting Lymph Formation and Muscle Compressibility in Skeletal Muscle During Contraction and Stretch
Quantitative model for predicting lymph formation and muscle compressibility in skeletal muscle during contraction and stretch Laura Causey, Stephen C. Cowin, and Sheldon Weinbaum1 Department of Biomedical Engineering, The City College of New York, New York, NY 10031 Contributed by Sheldon Weinbaum, April 20, 2012 (sent for review March 2, 2012) Skeletal muscle is widely perceived as nearly incompressible despite perimysial compartments. It is the purpose of this paper to develop the fact that blood and lymphatic vessels within the endomysial and a theoretical framework and simplified anatomical model for perimysial spaces undergo significant changes in diameter and performing just such an analysis. length during stretch and contraction. These fluid shifts between Perhaps the most extensive experimental investigation of the fascicle and interstitial compartments have proved extremely diffi- volume changes mentioned above is attributable to Mazzoni et al. cult to measure. In this paper, we propose a theoretical framework (5) who studied entire cross-sections of the rat spinotrapezius based on a space-filling hexagonal fascicle array to provide pre- muscle, a muscle comparable to a portion of the trapezius muscle in humans. These investigators made detailed measurements of dictions of the displacement of blood and lymph into and out of the fi muscle’s endomysium and perimysium during stretch and contrac- the cross-sectional area of individual muscle bers, the thickness tion. We also use this model to quantify the distribution of blood and of the interstitial (perimysial) space between fascicles, and the initial lymphatic (IL) vessels within a fascicle and its perimysial space cross-sectional area of the ILs in planes transverse to the primary using data for the rat spinotrapezius muscle.