Evolution of the Fungal Self-Fertile Reproductive Life Style from Self-Sterile Ancestors
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Evolution of Genetic Systems in Filamentous Ascomycetes
Evolution of Genetic Systems in Filamentous Ascomycetes Evolutie van genetische systemen in hyphenvormende zakjeszwammen 0000 0513 3836 Promotor: dr. R.F. Hoekstra hoogleraar in de populatie- en kwantitatieve genetica fjtfoiißi f ßin Maarten J. Nauta Evolution of Genetic Systems in Filamentous Ascomycetes Proefschrift ter verkrijging van de graad van doctor in de landbouw- en milieuwetenschappen op gezag van de rector magnificus, dr. C.M. Karssen, in het openbaar te verdedigen op woensdag 12januar i 1994 des namiddags te vier uur in de Aula van de Landbouwuniversiteit te Wageningen. 15 0 S(p^ZJ> These investigations were supported by the Netherlands Organization for Scientific Research (N.W.O.). BibUt/FHEEK LAMDbOirWUNIVERSITEJi. WAGE NINGE N CIP-GEGEVENS KONINKLIJKE BIBLIOTHEEK, DEN HAAG Nauta, Maarten J. Evolution of genetic systems in filamentous ascomycetes / Maarten J. Nauta. - [ S.l. : s.n.]. -111 . Thesis Wageningen. - With ref. - With summary in Dutch. ISBN 90-5485-199-6 Subject headings: population genetics / ascomycetes. omslagontwerp: Ernst van Cleef foto omslag: Barrages tussen verschillende stammen van Podospora anserina als gevolg van vegetatieve incompatibiliteit. (met dank aan Inge Haspels) aan mijn ouders Voorwoord Dit proefschrift is het resultaat van vier jaar onderzoek, verricht bij de vakgroep Erfelijkheidsleer van de Landbouwuniversiteit in Wageningen. In zekere zin valt zo'n proefschrift te vergelijken met een levend wezen. Uit de genetica is bekend dat de verschijningsvorm van elk levend wezen tot stand komt door een combinatie van erfelijke aanleg en invloeden uit de omgeving. Voor een proefschrift geldt eigenlijk hetzelfde: Zowel het werk van de auteur, als de bijdragen van zijn omgeving zijn onontbeerlijk om tot een verschijningsvorm te komen. -

Algal Sex Determination and the Evolution of Anisogamy James Umen, Susana Coelho
Algal Sex Determination and the Evolution of Anisogamy James Umen, Susana Coelho To cite this version: James Umen, Susana Coelho. Algal Sex Determination and the Evolution of Anisogamy. Annual Review of Microbiology, Annual Reviews, 2019, 73 (1), 10.1146/annurev-micro-020518-120011. hal- 02187088 HAL Id: hal-02187088 https://hal.sorbonne-universite.fr/hal-02187088 Submitted on 17 Jul 2019 HAL is a multi-disciplinary open access L’archive ouverte pluridisciplinaire HAL, est archive for the deposit and dissemination of sci- destinée au dépôt et à la diffusion de documents entific research documents, whether they are pub- scientifiques de niveau recherche, publiés ou non, lished or not. The documents may come from émanant des établissements d’enseignement et de teaching and research institutions in France or recherche français ou étrangers, des laboratoires abroad, or from public or private research centers. publics ou privés. Annu. Rev. Microbiol. 2019. 73:X–X https://doi.org/10.1146/annurev-micro-020518-120011 Copyright © 2019 by Annual Reviews. All rights reserved Umen • Coelho www.annualreviews.org • Algal Sexes and Mating Systems Algal Sex Determination and the Evolution of Anisogamy James Umen1 and Susana Coelho2 1Donald Danforth Plant Science Center, St. Louis, Missouri 63132, USA; email: [email protected] 2Sorbonne Université, UPMC Université Paris 06, CNRS, Algal Genetics Group, UMR 8227, Integrative Biology of Marine Models, Station Biologique de Roscoff, CS 90074, F-29688, Roscoff, France [**AU: Please write the entire affiliation in French or write it all in English, rather than a combination of English and French**] ; email: [email protected] Abstract Algae are photosynthetic eukaryotes whose taxonomic breadth covers a range of life histories, degrees of cellular and developmental complexity, and diverse patterns of sexual reproduction. -

Sex in the Extremes: Lichen-Forming Fungi
Mycologist, Volume 19, Part 2 May 2005. ©Cambridge University Press Printed in the United Kingdom. DOI: 10.1017/S0269915XO5002016 Sex in the extremes: lichen-forming fungi FABIAN A. SEYMOUR, PETER D. CRITTENDEN & PAUL S. DYER* School of Biology, University of Nottingham, University Park, Nottingham, NG7 2RD, UK. Tel. +44 (0) 115 9513203, Fax +44 (0) 115 9513251 E-mail: [email protected]; [email protected] *Corresponding Author Lichens are characteristically found in environments subject to extremes of temperature, desiccation and low nutrient status. Despite this sexual structures are often formed in abundance. The underlying mechanisms of sex in lichen-forming fungi are discussed, together with possible ecological reasons for the persistence of sexuality. Special features of lichen sex are highlighted including sex at the limits of life on earth in Antarctica, re-licheniza- tion following sex and dispersal, and the perennial nature of lichen fruiting bodies. Keywords: lichen, fungi, sex, breeding system, (98%) belonging to the Ascomycotina (Kirk et al., symbiosis, extreme environments, Antarctica 2001). They display a variety of morphologies, from flattened crust (crustose) or leafy (foliose) forms to Lichens - living together in a long-term relation- shrubby or pendulous fruticose types (Honegger, 2001) ship (Figs 3, 4, 7, 8). Lichens are seen as a textbook example of a successful Life in extreme environments mutualistic symbiosis. They consist of at least two A key characteristic of lichens is that they have a organisms: a fungus (the ‘mycobiont’), and an remarkable ability to tolerate extreme environmental intimately associated photosynthetic partner (the conditions and sustain growth despite frequent cycles ‘photobiont’). -
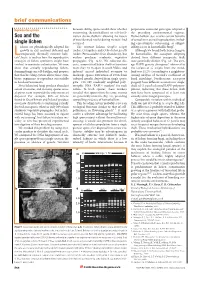
Brief Comms Layout 6/4Mx
brief communications Reproductive systems between sibling spores would show whether perpetuates successful genotypes adapted to outcrossing (heterothallism) or self-fertil- the prevailing environmental regimes. Sex and the ization (homothallism: allowing the fusion Homothallism also retains certain benefits of two identical nuclei during meiosis) had of sexual over asexual reproduction, includ- single lichen occurred. ing opportunistic outcrossing, as obligate ichens are physiologically adapted for The crustose lichens Graphis scripta selfing is rare in homothallic fungi8. growth in dry, nutrient-deficient and (order: Ostropales) and Ochrolechia parella Although we found both lichen fungi to Ltemporarily thermally extreme habi- (order: Pertusariales) fruit abundantly, but be homothallic, the ascospore offspring tats1, but it is unclear how the reproductive neither produces symbiotic vegetative derived from different conspecific thalli strategies of lichen symbionts might have propagules (Fig. 1a,b). We collected dis- were genetically distinct (Fig. 1d). The aver- evolved to maximize colonization. We now crete, symmetrical lichen thalli at locations age RAPD genetic divergence9 observed in show that sexually reproducing lichen- more than 10 m apart in south Wales, and five isolates of G. scripta from one wood- forming fungi can self-fertilize, and propose induced excised individual ascomata to land was 15.2% (according to a neighbour- that this breeding system allows these sym- discharge spores. Extraction of DNA from joining analysis of Jaccard’s coefficient of biotic organisms to reproduce successfully cultured mycelia derived from single spores band matching). Furthermore, ascospore in harsh environments. gave 218–263 randomly amplified poly- progeny from different ascomata on ‘single’ Most lichenized fungi produce abundant morphic DNA (RAPD) markers4 for each thalli of O. -

The Phylogeny of Plant and Animal Pathogens in the Ascomycota
Physiological and Molecular Plant Pathology (2001) 59, 165±187 doi:10.1006/pmpp.2001.0355, available online at http://www.idealibrary.com on MINI-REVIEW The phylogeny of plant and animal pathogens in the Ascomycota MARY L. BERBEE* Department of Botany, University of British Columbia, 6270 University Blvd, Vancouver, BC V6T 1Z4, Canada (Accepted for publication August 2001) What makes a fungus pathogenic? In this review, phylogenetic inference is used to speculate on the evolution of plant and animal pathogens in the fungal Phylum Ascomycota. A phylogeny is presented using 297 18S ribosomal DNA sequences from GenBank and it is shown that most known plant pathogens are concentrated in four classes in the Ascomycota. Animal pathogens are also concentrated, but in two ascomycete classes that contain few, if any, plant pathogens. Rather than appearing as a constant character of a class, the ability to cause disease in plants and animals was gained and lost repeatedly. The genes that code for some traits involved in pathogenicity or virulence have been cloned and characterized, and so the evolutionary relationships of a few of the genes for enzymes and toxins known to play roles in diseases were explored. In general, these genes are too narrowly distributed and too recent in origin to explain the broad patterns of origin of pathogens. Co-evolution could potentially be part of an explanation for phylogenetic patterns of pathogenesis. Robust phylogenies not only of the fungi, but also of host plants and animals are becoming available, allowing for critical analysis of the nature of co-evolutionary warfare. Host animals, particularly human hosts have had little obvious eect on fungal evolution and most cases of fungal disease in humans appear to represent an evolutionary dead end for the fungus. -

A New Species of Bipolaris from Heliconia Rostrata in India
Current Research in Environmental & Applied Mycology 6 (3): 231–237(2016) ISSN 2229-2225 www.creamjournal.org Article CREAM Copyright © 2016 Online Edition Doi 10.5943/cream/6/3/11 A new species of Bipolaris from Heliconia rostrata in India Singh R1 and Kumar S2 1Centre of Advanced Study in Botany, Banaras Hindu University, Varanasi – 221005, Uttar Pradesh, India 2Department of Forest Pathology, Kerala Forest Research Institute, Peechi- 680653, Kerala, India Singh R, Kumar S 2016 − A new species of Bipolaris from Heliconia rostrata in India. Current Research in Environmental & Applied Mycology 6(3), 231– 237, Doi 10.5943/cream/6/3/11 Abstract Bipolaris rostratae, a new foliicolous anamorphic fungus discovered on living leaves of Heliconia rostrata (Heliconiaceae), is described and illustrated. The species was compared with closely related species of Bipolaris and similar fungi recorded on Heliconia spp. This species is different from other Bipolaris spp. reported on Heliconia due to its shorter, thinner and less septate conidia. A key is provided to all species of Bipolaris reported on Heliconia. Key words − fungal diversity – morphotaxonomy – Foliicolous fungi – Bipolaris – new species Introduction After several taxonomic refinements, graminicolous Helminthosporium were segregated into several genera including Bipolaris, Curvularia, Drechslera and Exserohilum (Sivanesan 1987). These genera belong to Ascomycota, Dothideomycetes, Pleosporales, Pleosporaceae. These genera can be distinguished on the basis of characters such as conidial shape and size, hilum morphology, origin of the germ tubes from the basal or other conidial cells, and the location and sequence in the development of the conidial septa. Illustrations of different hilum morphologies in graminicolous Helminthosporium species were given by Alcorn (1988). -

The Classification of Lower Organisms
The Classification of Lower Organisms Ernst Hkinrich Haickei, in 1874 From Rolschc (1906). By permission of Macrae Smith Company. C f3 The Classification of LOWER ORGANISMS By HERBERT FAULKNER COPELAND \ PACIFIC ^.,^,kfi^..^ BOOKS PALO ALTO, CALIFORNIA Copyright 1956 by Herbert F. Copeland Library of Congress Catalog Card Number 56-7944 Published by PACIFIC BOOKS Palo Alto, California Printed and bound in the United States of America CONTENTS Chapter Page I. Introduction 1 II. An Essay on Nomenclature 6 III. Kingdom Mychota 12 Phylum Archezoa 17 Class 1. Schizophyta 18 Order 1. Schizosporea 18 Order 2. Actinomycetalea 24 Order 3. Caulobacterialea 25 Class 2. Myxoschizomycetes 27 Order 1. Myxobactralea 27 Order 2. Spirochaetalea 28 Class 3. Archiplastidea 29 Order 1. Rhodobacteria 31 Order 2. Sphaerotilalea 33 Order 3. Coccogonea 33 Order 4. Gloiophycea 33 IV. Kingdom Protoctista 37 V. Phylum Rhodophyta 40 Class 1. Bangialea 41 Order Bangiacea 41 Class 2. Heterocarpea 44 Order 1. Cryptospermea 47 Order 2. Sphaerococcoidea 47 Order 3. Gelidialea 49 Order 4. Furccllariea 50 Order 5. Coeloblastea 51 Order 6. Floridea 51 VI. Phylum Phaeophyta 53 Class 1. Heterokonta 55 Order 1. Ochromonadalea 57 Order 2. Silicoflagellata 61 Order 3. Vaucheriacea 63 Order 4. Choanoflagellata 67 Order 5. Hyphochytrialea 69 Class 2. Bacillariacea 69 Order 1. Disciformia 73 Order 2. Diatomea 74 Class 3. Oomycetes 76 Order 1. Saprolegnina 77 Order 2. Peronosporina 80 Order 3. Lagenidialea 81 Class 4. Melanophycea 82 Order 1 . Phaeozoosporea 86 Order 2. Sphacelarialea 86 Order 3. Dictyotea 86 Order 4. Sporochnoidea 87 V ly Chapter Page Orders. Cutlerialea 88 Order 6. -

Multiple Pathways to Homothallism in Closely Related Yeast Lineages in the Basidiomycota 2 3 Alexandra Cabrita1, Márcia David-Palma1*, Patrícia H
bioRxiv preprint doi: https://doi.org/10.1101/2020.09.30.320192; this version posted December 21, 2020. The copyright holder for this preprint (which was not certified by peer review) is the author/funder, who has granted bioRxiv a license to display the preprint in perpetuity. It is made available under aCC-BY-ND 4.0 International license. 1 Multiple pathways to homothallism in closely related yeast lineages in the Basidiomycota 2 3 Alexandra Cabrita1, Márcia David-Palma1*, Patrícia H. Brito1, Joseph Heitman2, Marco A. Coelho1* and 4 Paula Gonçalves1# 5 # Corresponding author: [email protected] 6 1. Applied Molecular Biosciences Unit-UCIBIO, Departamento de Ciências da Vida, Faculdade de 7 Ciências e Tecnologia, Universidade Nova de Lisboa, 2829-516 Caparica, Portugal 8 2. Department of Molecular Genetics and Microbiology, Duke University, Duke University Medical 9 Center, Durham, NC 27710, USA. 10 Running title: Homothallism in Cystofilobasidium 11 12 Abstract 13 Sexual reproduction in fungi relies on proteins with well-known functions encoded by the mating-type 14 (MAT) loci. In the Basidiomycota, MAT loci are often bipartite, with the P/R locus encoding pheromone 15 precursors and pheromone receptors and the HD locus encoding heterodimerizing homeodomain 16 transcription factors (Hd1/Hd2). The interplay between different alleles of these genes within a single 17 species usually generates at least two compatible mating types. However, a minority of species are 18 homothallic, reproducing sexually without an obligate need for a compatible partner. Here we examine 19 the organization and function of the MAT loci of Cystofilobasidium capitatum, a species in the order 20 Cystofilobasidiales, which is unusually rich in homothallic species. -

Phytotoxin HC-Toxin (Cyclic Peptide Biosynthesis/Maize/Plant Disease) JONATHAN D
Proc. Nati. Acad. Sci. USA Vol. 84, pp. 8444-8447, December 1987 Botany Two enzymes involved in biosynthesis of the host-selective phytotoxin HC-toxin (cyclic peptide biosynthesis/maize/plant disease) JONATHAN D. WALTON Department of Energy Plant Research Laboratory, Michigan State University, East Lansing, MI 48824 Communicated by Anton Lang, August 24, 1987 (receivedfor review June 2, 1987) ABSTRACT Cochliobolus carbonum race 1 produces a C. carbonum (imperfect stage Helminthosporium carbo- cyclic tetrapeptide HC-toxin, which is necessary for its excep- num or Bipolaris zeicola) race 1 synthesizes the host- tional virulence on certain varieties of maize. Previous genetic selective toxin HC-toxin (8-12). HC-toxin is a cyclic peptide analysis of HC-toxin production by the fungus has indicated with the structure cyclo(D-Pro-L-Ala-D-Ala-L-AOE), where that a single genetic locus controls HC-toxin production. AOE stands for 2-amino-8-oxo-9,10-epoxidecanoic acid (Fig. Enzymes involved in the biosynthesis of HC-toxin have been 1). The unusual amino acid AOE has been reported in three sought by following the precedents established for the biosyn- other cyclic tetrapeptides from three unrelated filamentous thetic enzymes of cyclic peptide antibiotics. Two enzymatic fungi (13-15). Maize (Zea mays L.) that is homozygous activities from C. carbonum race 1 were found, a D-alanine- and recessive at the nuclear Hm locus is susceptible to C. an L-proline-dependent ATP/PPj exchange, which by biochem- carbonum race 1 and sensitive to HC-toxin. Non-toxin- ical and genetic criteria were shown to be involved in the producing isolates (races 2 and 3) of C. -

(Bplb) INFECTED WHEAT LEAVES Department of Botany, Un
J. Bangladesh Acad. Sci., Vol. 43, No. 1, 11-16, 2019 DOI: https://doi.org/10.3329/jbas.v43i1.42228 ASSOCIATION OF BIPOLARIS AND DRECHSLERA SPECIES WITH BIPOLARIS LEAF BLIGHT (BpLB) INFECTED WHEAT LEAVES MST. SELINA MOMTAZ1, SHAMIM SHAMSI* AND TAPAN KUMAR DEY2 Department of Botany, University of Dhaka, Dhaka-1000, Bangladesh ABSTRACT Five species of Bipolaris and two species of Drechslera associated with leaf blight disease of wheat (Triticum aestivum L.) have been described. The associated fungi were Bipolaris cynodontis (Marig.) Shoemaker, B. oryzae (Breda De Haan) Shoemaker, B. sorokiniana (Sacc.) Shoemaker, B. tetramera (Mckinney) Shoemaker, B. victoriae (Meehan & Murphy) Shoemaker, Drechslera dematioidea (Bub. & Wrob.) Subram. & Jain and D. hawaiiensis (Bugnicourt) ex M.B. Ellis; Subram. & Jain. Keywords: Wheat; Bipolaris; Drechslera; BpLB INTRODUCTION Wheat (Triticum aestivum L.) is the second most The present study was on BpLB or Bipolaris leaf important staple food crop in Bangladesh after rice. blight disease of wheat caused by Bipolaris Once wheat was a food for the poorer in sorokiniana (Sacc.) Shoemaker (syn. Bangladesh. Most of the people used to take wheat Helminthosporium sativum PK & B; teliomorph: as ‘roti’. Wheat consumption is increasing due to Cochliobolus sativus Ito & Kurib; Drechslera rapid urbanization and industrialization of the sorokiniana Drechs ex Dastur). Shoemaker country and the consequent increase in the use of (1959, 1962) proposed the generic name numerous bakery products. Within a period of 40 Bipolaris for the Helminthosporium species with years of time, wheat has been firmly established as a fusoid, straight, or curved conidia germinating secure crop in Bangladesh. The average yield of by one germ tube from each end (bipolar wheat in Bangladesh is lower in comparison to other germination). -

Sex, Outcrossing and Mating Types: Unsolved Questions in Fungi and Beyond
doi: 10.1111/j.1420-9101.2012.02495.x REVIEW Sex, outcrossing and mating types: unsolved questions in fungi and beyond S. BILLIARD*, M. LO´ PEZ-VILLAVICENCIO ,M.E.HOODà &T.GIRAUD§– *Laboratoire de Ge´ne´tique et Evolution des Populations Ve´ge´tales, UMR CNRS 8016, Universite´ des Sciences et Technologies de Lille – Lille1, Villeneuve d’Ascq Cedex, France Origine, Structure, Evolution de la Biodiversite´, UMR 7205 CNRS-MNHN, Muse´um National d’Histoire Naturelle, Paris Cedex, France àDepartment of Biology, McGuire Life Sciences Building, Amherst College, Amherst, MA, USA §Ecologie, Syste´matique et Evolution, Universite´ Paris-Sud, Orsay cedex, France –Ecologie, Syste´matique et Evolution, CNRS, Orsay cedex, France Keywords: Abstract ascomycete; Variability in the way organisms reproduce raises numerous, and still asexual reproduction; unsolved, questions in evolutionary biology. In this study, we emphasize that basidiomycete; fungi deserve a much greater emphasis in efforts to address these questions breeding systems; because of their multiple advantages as model eukaryotes. A tremendous diploid selfing; diversity of reproductive modes and mating systems can be found in fungi, gametophytic selfing; with many evolutionary transitions among closely related species. In addition, haploid selfing; fungi show some peculiarities in their mating systems that have received little mating systems; attention so far, despite the potential for providing insights into important oomycetes; evolutionary questions. In particular, selfing can occur at the haploid stage in sexual reproduction. addition to the diploid stage in many fungi, which is generally not possible in animals and plants but has a dramatic influence upon the structure of genetic systems. Fungi also present several advantages that make them tractable models for studies in experimental evolution. -

Oxygen, Life Forms, and the Evolution of Sexes in Multicellular Eukaryotes
Heredity (2020) 125:1–14 https://doi.org/10.1038/s41437-020-0317-9 REVIEW ARTICLE Oxygen, life forms, and the evolution of sexes in multicellular eukaryotes 1 2 Elvira Hörandl ● Franz Hadacek Received: 24 September 2019 / Revised: 26 April 2020 / Accepted: 26 April 2020 / Published online: 15 May 2020 © The Author(s) 2020. This article is published with open access Abstract The evolutionary advantage of different sexual systems in multicellular eukaryotes is still not well understood, because the differentiation into male and female individuals halves offspring production compared with asexuality. Here we propose that various physiological adaptations to oxidative stress could have forged sessility versus motility, and consequently the evolution of sexual systems in multicellular animals, plants, and fungi. Photosynthesis causes substantial amounts of oxidative stress in photoautotrophic plants and, likewise, oxidative chemistry of polymer breakdown, cellulose and lignin, for saprotrophic fungi. In both cases, its extent precludes motility, an additional source of oxidative stress. Sessile life form and the lack of neuronal systems, however, limit options for mate recognition and adult sexual selection, resulting in fi 1234567890();,: 1234567890();,: inef cient mate-searching systems. Hence, sessility requires that all individuals can produce offspring, which is achieved by hermaphroditism in plants and/or by multiple mating types in fungi. In animals, motility requires neuronal systems, and muscle activity, both of which are highly sensitive to oxidative damage. As a consequence, motility has evolved in animals as heterotrophic organisms that (1) are not photosynthetically active, and (2) are not primary decomposers. Adaptations to motility provide prerequisites for an active mating behavior and efficient mate-searching systems.