Evolution of Genetic Systems in Filamentous Ascomycetes
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Algal Sex Determination and the Evolution of Anisogamy James Umen, Susana Coelho
Algal Sex Determination and the Evolution of Anisogamy James Umen, Susana Coelho To cite this version: James Umen, Susana Coelho. Algal Sex Determination and the Evolution of Anisogamy. Annual Review of Microbiology, Annual Reviews, 2019, 73 (1), 10.1146/annurev-micro-020518-120011. hal- 02187088 HAL Id: hal-02187088 https://hal.sorbonne-universite.fr/hal-02187088 Submitted on 17 Jul 2019 HAL is a multi-disciplinary open access L’archive ouverte pluridisciplinaire HAL, est archive for the deposit and dissemination of sci- destinée au dépôt et à la diffusion de documents entific research documents, whether they are pub- scientifiques de niveau recherche, publiés ou non, lished or not. The documents may come from émanant des établissements d’enseignement et de teaching and research institutions in France or recherche français ou étrangers, des laboratoires abroad, or from public or private research centers. publics ou privés. Annu. Rev. Microbiol. 2019. 73:X–X https://doi.org/10.1146/annurev-micro-020518-120011 Copyright © 2019 by Annual Reviews. All rights reserved Umen • Coelho www.annualreviews.org • Algal Sexes and Mating Systems Algal Sex Determination and the Evolution of Anisogamy James Umen1 and Susana Coelho2 1Donald Danforth Plant Science Center, St. Louis, Missouri 63132, USA; email: [email protected] 2Sorbonne Université, UPMC Université Paris 06, CNRS, Algal Genetics Group, UMR 8227, Integrative Biology of Marine Models, Station Biologique de Roscoff, CS 90074, F-29688, Roscoff, France [**AU: Please write the entire affiliation in French or write it all in English, rather than a combination of English and French**] ; email: [email protected] Abstract Algae are photosynthetic eukaryotes whose taxonomic breadth covers a range of life histories, degrees of cellular and developmental complexity, and diverse patterns of sexual reproduction. -

Evolutionary Pathways to Self-Fertilization in a Tristylous Plant
Review BlackwellOxford,NPHNew0028-646X1469-8137©293710.1111/j.1469-8137.2009.02937.xJune0546???556???ResearchXX The 2009Phytologist Authors UK Review Publishing (2009). Ltd Journal compilation © New Phytologist (2009) Research reviewXX Evolutionary pathways to self- fertilization in a tristylous plant species Author for correspondence: Spencer C. H. Barrett, Rob W. Ness and Mario Vallejo-Marín Spencer C. H. Barrett Department of Ecology and Evolutionary Biology, University of Toronto, 25 Willcocks St, Toronto, Tel: +1 416 978 5603 Email: [email protected] Ontario, Canada, M5S 3B2 Received: 23 April 2009 Accepted: 20 May 2009 Summary New Phytologist (2009) 183: 546–556 Evolutionary transitions from outcrossing to selfing occur commonly in heterostylous doi: 10.1111/j.1469-8137.2009.02937.x genera. The morphological polymorphisms that characterize heterostyly provide opportunities for different pathways for selfing to evolve. Here, we investigate the Key words: developmental instability, origins and pathways by which selfing has evolved in tristylous Eichhornia paniculata Eichhornia, herkogamy, heterostyly, multiple by providing new evidence based on morphology, DNA sequences and genetic analysis. origins, pathways to self-fertilization. The primary pathway from outcrossing to selfing involves the stochastic loss of the short-styled morph (S-morph) from trimorphic populations, followed by the spread of selfing variants of the mid-styled morph (M-morph). However, the discovery of selfing variants of the long-styled morph (L-morph) in Central America indicates a secondary pathway and distinct origin for selfing. Comparisons of multi-locus nucleo- tide sequences from 27 populations sampled from throughout the geographical range suggest multiple transitions to selfing. Genetic analysis of selfing variants of the L- and M-morphs demonstrates recessive control of the loss of herkogamy, although the number of factors appears to differ between the forms. -

Self-Fertility and Uni-Directional Mating-Type Switching in Ceratocystis Coerulescens, a Filamentous Ascomycete
Curr Genet (1997) 32: 52–59 © Springer-Verlag 1997 ORIGINAL PAPER T. C. Harrington · D. L. McNew Self-fertility and uni-directional mating-type switching in Ceratocystis coerulescens, a filamentous ascomycete Received: 6 July 1996 / 25 March 1997 Abstract Individual perithecia from selfings of most some filamentous ascomycetes. Although a switch in the Ceratocystis species produce both self-fertile and self- expression of mating-type is seen in these fungi, it is not sterile progeny, apparently due to uni-directional mating- clear if a physical movement of mating-type genes is in- type switching. In C. coerulescens, male-only mutants of volved. It is also not clear if the expressed mating-types otherwise hermaphroditic and self-fertile strains were self- of the respective self-fertile and self-sterile progeny are sterile and were used in crossings to demonstrate that this homologs of the mating-type genes in other strictly heter- species has two mating-types. Only MAT-2 strains are othallic species of ascomycetes. capable of selfing, and half of the progeny from a MAT-2 Sclerotinia trifoliorum and Chromocrea spinulosa show selfing are MAT-1. Male-only, MAT-2 mutants are self- a 1:1 segregation of self-fertile and self-sterile progeny in sterile and cross only with MAT-1 strains. Similarly, self- perithecia from selfings or crosses (Mathieson 1952; Uhm fertile strains generally cross with only MAT-1 strains. and Fujii 1983a, b). In tetrad analyses of selfings or crosses, MAT-1 strains only cross with MAT-2 strains and never self. half of the ascospores in an ascus are large and give rise to It is hypothesized that the switch in mating-type during self-fertile colonies, and the other ascospores are small and selfing is associated with a deletion of the MAT-2 gene. -

Genetic Diversity in Partially Selfing Populations with the Stepping-Stone Structure
Heredity 77 (1996) 469—475 Received 20 October 1995 Genetic diversity in partially selfing populations with the stepping-stone structure HIDENORI TACHIDA*t & HIROSHI YOSHIMARU tDepartment of Biology, Faculty of Science, Kyushu University 33, Fukuoka 812, Japan and tPopulation Genetics Laboratory, Forestry and Forest Products Research Institute, Tsukuba, Ibaraki 305, Japan Amethod to compute identity coefficients of two genes in the stepping-stone model with partial selfing is developed. The identity coefficients in partially selfing populations are computed from those in populations without selfing as functions of s (selfing rate), m (migra- tion rate), N (subpopulation size), n (number of subpopulations) and u (mutation rate). For small m, 1/N and u, it is shown that approximate formulae for the identity coefficients of two genes from different individuals are the same as those in random mating populations if we replace N in the latter with N(1 —s12).Thus,the effects of selfing on genetic variability are summarized as reducing variation within subpopulations and increasing differentiation among subpopulations by reducing the subpopulation size. The extent of biparental inbreeding as measured by the genotypic correlation between truly outcrossed mates was computed in the one-dimensional stepping-stone model. The correlation was shown to be independent of the selfing rate and starts to fall off as the migration rate increases when mN is larger than 0.1. Keywords:biparentalinbreeding, fixation index, genetic variability, geographical differentia- tion, selfing, stepping-stone model. Introduction Model analysis Manyplant species are partially self-fertilizing Genesare defined to be identical by descent if they (Brown, 1990; Murawski & Hamrick, 1991, 1992; have a common ancestor gene and there is no muta- Kitamura et a!., 1994). -

Sex in the Extremes: Lichen-Forming Fungi
Mycologist, Volume 19, Part 2 May 2005. ©Cambridge University Press Printed in the United Kingdom. DOI: 10.1017/S0269915XO5002016 Sex in the extremes: lichen-forming fungi FABIAN A. SEYMOUR, PETER D. CRITTENDEN & PAUL S. DYER* School of Biology, University of Nottingham, University Park, Nottingham, NG7 2RD, UK. Tel. +44 (0) 115 9513203, Fax +44 (0) 115 9513251 E-mail: [email protected]; [email protected] *Corresponding Author Lichens are characteristically found in environments subject to extremes of temperature, desiccation and low nutrient status. Despite this sexual structures are often formed in abundance. The underlying mechanisms of sex in lichen-forming fungi are discussed, together with possible ecological reasons for the persistence of sexuality. Special features of lichen sex are highlighted including sex at the limits of life on earth in Antarctica, re-licheniza- tion following sex and dispersal, and the perennial nature of lichen fruiting bodies. Keywords: lichen, fungi, sex, breeding system, (98%) belonging to the Ascomycotina (Kirk et al., symbiosis, extreme environments, Antarctica 2001). They display a variety of morphologies, from flattened crust (crustose) or leafy (foliose) forms to Lichens - living together in a long-term relation- shrubby or pendulous fruticose types (Honegger, 2001) ship (Figs 3, 4, 7, 8). Lichens are seen as a textbook example of a successful Life in extreme environments mutualistic symbiosis. They consist of at least two A key characteristic of lichens is that they have a organisms: a fungus (the ‘mycobiont’), and an remarkable ability to tolerate extreme environmental intimately associated photosynthetic partner (the conditions and sustain growth despite frequent cycles ‘photobiont’). -

Inbreeding Depression in Two Populations of Arenaria Uni¯Ora (Caryophyllaceae) with Contrasting Mating Systems
Heredity 86 (2001) 184±194 Received 16 November 1999, accepted 17 October 2000 Inbreeding depression in two populations of Arenaria uni¯ora (Caryophyllaceae) with contrasting mating systems LILA FISHMAN Department of Ecology and Evolutionary Biology, Princeton University, Princeton, NJ 08544 U.S.A. I used parallel family-structured crossing designs to investigate the relative performance of self and outcross progeny in sel®ng and predominantly outcrossing populations of the annual plant Arenaria uni¯ora. The selfer population experienced much lower inbreeding depression (d 0.05 0.02 SE) than the outcrossers (d 0.19 0.02 SE). The negative association between genetic load and sel®ng rate suggests that purgable partially recessive alleles are the primary source of inbreeding depression, as does its late expression in both populations. Inbreeding depression in the selfer population, which naturally consists of highly inbred lines, was used to calculate the mean dominance (h 0.33) and incidence rate (U 0.30) of deleterious mutations. In the outcrosser population, signi®cant variation among individuals in the expression of inbreeding depression may re¯ect lineage-speci®c dierences in inbreeding history or, more probably, random variation in mutational load. The low (0.5) inbreeding depression of outcrossers suggests that the maintenance of a mixed mating system in some A. uni¯ora populations and the evolution of nearly cleistogamous self-pollination in others may re¯ect local pollinator-mediated selection for sel®ng rather than the constant 3:2 genetic advantage invoked by many models. Keywords: Arenaria uni¯ora, genetic load, inbreeding depression, mating system, sel®ng. -

Population Genetics of the Wild Yeast Saccharomyces Paradoxus
Copyright 2004 by the Genetics Society of America Population Genetics of the Wild Yeast Saccharomyces paradoxus Louise J. Johnson,*,1 Vassiliki Koufopanou,* Matthew R. Goddard,† Richard Hetherington,* Stefanie M. Scha¨fer*,2 and Austin Burt* *Department of Biological Sciences and †NERC Centre for Population Biology, Imperial College at Silwood Park, Ascot SL5 7PY, United Kingdom Manuscript received November 4, 2002 Accepted for publication September 22, 2003 ABSTRACT Saccharomyces paradoxus is the closest known relative of the well-known S. cerevisiae and an attractive model organism for population genetic and genomic studies. Here we characterize a set of 28 wild isolates from a 10-km2 sampling area in southern England. All 28 isolates are homothallic (capable of mating-type switching) and wild type with respect to nutrient requirements. Nine wild isolates and two lab strains of S. paradoxus were surveyed for sequence variation at six loci totaling 7 kb, and all 28 wild isolates were then genotyped at seven polymorphic loci. These data were used to calculate nucleotide diversity and number of segregating sites in S. paradoxus and to investigate geographic differentiation, population Extensive incompatibilities .%0.3ف structure, and linkage disequilibrium. Synonymous site diversity is between gene genealogies indicate frequent recombination between unlinked loci, but there is no evidence of recombination within genes. Some localized clonal growth is apparent. The frequency of outcrossing relative to inbreeding is estimated at 1.1% on the basis of heterozygosity. Thus, all three modes of reproduction known in the lab (clonal replication, inbreeding, and outcrossing) have been important in molding genetic variation in this species. -
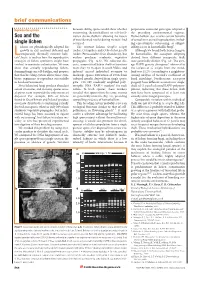
Brief Comms Layout 6/4Mx
brief communications Reproductive systems between sibling spores would show whether perpetuates successful genotypes adapted to outcrossing (heterothallism) or self-fertil- the prevailing environmental regimes. Sex and the ization (homothallism: allowing the fusion Homothallism also retains certain benefits of two identical nuclei during meiosis) had of sexual over asexual reproduction, includ- single lichen occurred. ing opportunistic outcrossing, as obligate ichens are physiologically adapted for The crustose lichens Graphis scripta selfing is rare in homothallic fungi8. growth in dry, nutrient-deficient and (order: Ostropales) and Ochrolechia parella Although we found both lichen fungi to Ltemporarily thermally extreme habi- (order: Pertusariales) fruit abundantly, but be homothallic, the ascospore offspring tats1, but it is unclear how the reproductive neither produces symbiotic vegetative derived from different conspecific thalli strategies of lichen symbionts might have propagules (Fig. 1a,b). We collected dis- were genetically distinct (Fig. 1d). The aver- evolved to maximize colonization. We now crete, symmetrical lichen thalli at locations age RAPD genetic divergence9 observed in show that sexually reproducing lichen- more than 10 m apart in south Wales, and five isolates of G. scripta from one wood- forming fungi can self-fertilize, and propose induced excised individual ascomata to land was 15.2% (according to a neighbour- that this breeding system allows these sym- discharge spores. Extraction of DNA from joining analysis of Jaccard’s coefficient of biotic organisms to reproduce successfully cultured mycelia derived from single spores band matching). Furthermore, ascospore in harsh environments. gave 218–263 randomly amplified poly- progeny from different ascomata on ‘single’ Most lichenized fungi produce abundant morphic DNA (RAPD) markers4 for each thalli of O. -

MEIOSIS and RECOMBINATION in SORDARIA FIMICOLA Introduction
MEIOSIS AND RECOMBINATION IN SORDARIA FIMICOLA Introduction: In ascomycete fungi, a form of meiosis occurs in which the products of meiosis order themselves within a fruiting body according to the physical separation and segregation of chromatids during the meiotic process. This is covered in some detail on pages 150-152 (including Figures 4.26 and 4.27) in Hartl and Jones, Essential Genetics. You should study these pages before beginning this module. As described, ordered tetrad analysis provides a way to measure the genetic map distance between a gene and the centromere of the chromosome on which that gene resides. That is what you will do over the next two weeks in this laboratory. I. Natural history and Life Cycle of Sordaria fimicola Sordaria fimicola is an ascomycete fungi that can be found growing in rotting vegetation and animal dung (in fact, the name Sordaria fimicola means "filthy dung dweller"). Sordaria and another ascomycete, the common bread fungus Neurospora crassa (Fig. 4.26), have been used as model systems for studying the process of chromosome exchange (crossing-over) because of their reproductive characteristics. The life cycle of Sordaria is representative of the ascomycetes (although there are substantial differences in the details among species). The individual fungus begins as a haploid ascospore. The ascospore germinates to form hyphae (singular = hypha), which are long filaments comprised of haploid cells. These hyphae grow and extend throughout the nutrient source (dung or rotting vegetation in nature, nutrient medium in the laboratory situation) and digest it by means of enzymes secreted by the cells. Nutrients are then absorbed into the cells. -

Advanced Gene Mapping in Eukaryotes
RUSSMC07 2/7/05 11:54 AM Page 165 7 Advanced Gene Mapping in Eukaryotes Ordered tetrads of the fungus, Neurospora Crassa. PRINCIPAL POINTS • Tetrad analysis is a mapping technique that can be be used to determine gene order and map distances used to map the genes of certain haploid eukaryotic between genes. organisms in which the products of a single meiosis— • Due to both ethical and practical issues, human the meiotic tetrad—are contained within a single genes cannot be mapped by making crosses and structure. In these situations, the map distance analyzing progeny. A number of approaches are between genes is computed by analyzing the relative used to determine the linkage relationships proportion of tetrad types, rather than by analyzing between human genes, including analyzing individual progeny. pedigree data recombinationally and physically • In organisms in which the meiotic tetrads are locating genes on chromosomes by molecularly arranged linearly, it is easy to map the distance aided methods. of a gene from its centromere. • Crossing-over can also occur during mitosis, although i SPECIAL TECHNIQUES ARE AVAILABLE FOR at a frequency much lower than during meiosis. As in constructing linkage maps for eukaryotic organisms meiosis, mitotic crossing-over occurs at a four-strand beyond the standard genetic mapping crosses. In this stage. chapter you will learn about these techniques. Then, in the iActivity, you can apply what you’ve learned and further • For organisms amenable to such analysis, such as the explore one of the advanced mapping techniques. fungus Aspergillus nidulans, mitotic recombination can iActivity 165 RUSSMC07 1/26/05 12:47 PM Page 166 166 Chapter 7 Advanced Gene Mapping in Eukaryotes In the previous chapter, we considered the classical princi- Go to the iActivity Mapping Genes by Tetrad i ples for mapping genes in eukaryotes by means of recombi- Analysis on your CD-ROM and assume the role nation analysis. -

The Classification of Lower Organisms
The Classification of Lower Organisms Ernst Hkinrich Haickei, in 1874 From Rolschc (1906). By permission of Macrae Smith Company. C f3 The Classification of LOWER ORGANISMS By HERBERT FAULKNER COPELAND \ PACIFIC ^.,^,kfi^..^ BOOKS PALO ALTO, CALIFORNIA Copyright 1956 by Herbert F. Copeland Library of Congress Catalog Card Number 56-7944 Published by PACIFIC BOOKS Palo Alto, California Printed and bound in the United States of America CONTENTS Chapter Page I. Introduction 1 II. An Essay on Nomenclature 6 III. Kingdom Mychota 12 Phylum Archezoa 17 Class 1. Schizophyta 18 Order 1. Schizosporea 18 Order 2. Actinomycetalea 24 Order 3. Caulobacterialea 25 Class 2. Myxoschizomycetes 27 Order 1. Myxobactralea 27 Order 2. Spirochaetalea 28 Class 3. Archiplastidea 29 Order 1. Rhodobacteria 31 Order 2. Sphaerotilalea 33 Order 3. Coccogonea 33 Order 4. Gloiophycea 33 IV. Kingdom Protoctista 37 V. Phylum Rhodophyta 40 Class 1. Bangialea 41 Order Bangiacea 41 Class 2. Heterocarpea 44 Order 1. Cryptospermea 47 Order 2. Sphaerococcoidea 47 Order 3. Gelidialea 49 Order 4. Furccllariea 50 Order 5. Coeloblastea 51 Order 6. Floridea 51 VI. Phylum Phaeophyta 53 Class 1. Heterokonta 55 Order 1. Ochromonadalea 57 Order 2. Silicoflagellata 61 Order 3. Vaucheriacea 63 Order 4. Choanoflagellata 67 Order 5. Hyphochytrialea 69 Class 2. Bacillariacea 69 Order 1. Disciformia 73 Order 2. Diatomea 74 Class 3. Oomycetes 76 Order 1. Saprolegnina 77 Order 2. Peronosporina 80 Order 3. Lagenidialea 81 Class 4. Melanophycea 82 Order 1 . Phaeozoosporea 86 Order 2. Sphacelarialea 86 Order 3. Dictyotea 86 Order 4. Sporochnoidea 87 V ly Chapter Page Orders. Cutlerialea 88 Order 6. -

Clonal Reproduction in Fungi COLLOQUIUM
PAPER Clonal reproduction in fungi COLLOQUIUM John W. Taylora,1, Christopher Hann-Sodena, Sara Brancoa, Iman Sylvaina, and Christopher E. Ellisonb Departments of aPlant and Microbial Biology and bIntegrative Biology, University of California, Berkeley, CA 94720 Edited by John C. Avise, University of California, Irvine, CA, and approved April 2, 2015 (received for review February 17, 2015) Research over the past two decades shows that both recombina- Clearly, mycologists have more work to do with these extremely tion and clonality are likely to contribute to the reproduction of all important fungi. fungi. This view of fungi is different from the historical and still commonly held view that a large fraction of fungi are exclusively Population Genetic Evidence for Recombination clonal and that some fungi have been exclusively clonal for Evidence that clonality was not limited to Glomales but instead hundreds of millions of years. Here, we first will consider how was widespread in fungi came from the oft-cited statistic that these two historical views have changed. Then we will examine 20% of fungi are asexual (12). This fraction represented the the impact on fungal research of the concept of restrained re- number of fungi for which sexual reproduction had not been combination [Tibayrenc M, Ayala FJ (2012) Proc Natl Acad Sci USA observed or was rarely observed. At a time when observation of 109 (48):E3305–E3313]. Using animal and human pathogenic fungi, the sexual morphology of a fungus was required for its classifi- we examine extrinsic restraints on recombination associated with cation, these fungi were classified in the Deuteromycota, apart bottlenecks in genetic variation caused by geographic dispersal from sexual fungi.