Sex, Outcrossing and Mating Types: Unsolved Questions in Fungi and Beyond
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Evolution of Genetic Systems in Filamentous Ascomycetes
Evolution of Genetic Systems in Filamentous Ascomycetes Evolutie van genetische systemen in hyphenvormende zakjeszwammen 0000 0513 3836 Promotor: dr. R.F. Hoekstra hoogleraar in de populatie- en kwantitatieve genetica fjtfoiißi f ßin Maarten J. Nauta Evolution of Genetic Systems in Filamentous Ascomycetes Proefschrift ter verkrijging van de graad van doctor in de landbouw- en milieuwetenschappen op gezag van de rector magnificus, dr. C.M. Karssen, in het openbaar te verdedigen op woensdag 12januar i 1994 des namiddags te vier uur in de Aula van de Landbouwuniversiteit te Wageningen. 15 0 S(p^ZJ> These investigations were supported by the Netherlands Organization for Scientific Research (N.W.O.). BibUt/FHEEK LAMDbOirWUNIVERSITEJi. WAGE NINGE N CIP-GEGEVENS KONINKLIJKE BIBLIOTHEEK, DEN HAAG Nauta, Maarten J. Evolution of genetic systems in filamentous ascomycetes / Maarten J. Nauta. - [ S.l. : s.n.]. -111 . Thesis Wageningen. - With ref. - With summary in Dutch. ISBN 90-5485-199-6 Subject headings: population genetics / ascomycetes. omslagontwerp: Ernst van Cleef foto omslag: Barrages tussen verschillende stammen van Podospora anserina als gevolg van vegetatieve incompatibiliteit. (met dank aan Inge Haspels) aan mijn ouders Voorwoord Dit proefschrift is het resultaat van vier jaar onderzoek, verricht bij de vakgroep Erfelijkheidsleer van de Landbouwuniversiteit in Wageningen. In zekere zin valt zo'n proefschrift te vergelijken met een levend wezen. Uit de genetica is bekend dat de verschijningsvorm van elk levend wezen tot stand komt door een combinatie van erfelijke aanleg en invloeden uit de omgeving. Voor een proefschrift geldt eigenlijk hetzelfde: Zowel het werk van de auteur, als de bijdragen van zijn omgeving zijn onontbeerlijk om tot een verschijningsvorm te komen. -

Algal Sex Determination and the Evolution of Anisogamy James Umen, Susana Coelho
Algal Sex Determination and the Evolution of Anisogamy James Umen, Susana Coelho To cite this version: James Umen, Susana Coelho. Algal Sex Determination and the Evolution of Anisogamy. Annual Review of Microbiology, Annual Reviews, 2019, 73 (1), 10.1146/annurev-micro-020518-120011. hal- 02187088 HAL Id: hal-02187088 https://hal.sorbonne-universite.fr/hal-02187088 Submitted on 17 Jul 2019 HAL is a multi-disciplinary open access L’archive ouverte pluridisciplinaire HAL, est archive for the deposit and dissemination of sci- destinée au dépôt et à la diffusion de documents entific research documents, whether they are pub- scientifiques de niveau recherche, publiés ou non, lished or not. The documents may come from émanant des établissements d’enseignement et de teaching and research institutions in France or recherche français ou étrangers, des laboratoires abroad, or from public or private research centers. publics ou privés. Annu. Rev. Microbiol. 2019. 73:X–X https://doi.org/10.1146/annurev-micro-020518-120011 Copyright © 2019 by Annual Reviews. All rights reserved Umen • Coelho www.annualreviews.org • Algal Sexes and Mating Systems Algal Sex Determination and the Evolution of Anisogamy James Umen1 and Susana Coelho2 1Donald Danforth Plant Science Center, St. Louis, Missouri 63132, USA; email: [email protected] 2Sorbonne Université, UPMC Université Paris 06, CNRS, Algal Genetics Group, UMR 8227, Integrative Biology of Marine Models, Station Biologique de Roscoff, CS 90074, F-29688, Roscoff, France [**AU: Please write the entire affiliation in French or write it all in English, rather than a combination of English and French**] ; email: [email protected] Abstract Algae are photosynthetic eukaryotes whose taxonomic breadth covers a range of life histories, degrees of cellular and developmental complexity, and diverse patterns of sexual reproduction. -

Purebred Dog Breeds Into the Twenty-First Century: Achieving Genetic Health for Our Dogs
Purebred Dog Breeds into the Twenty-First Century: Achieving Genetic Health for Our Dogs BY JEFFREY BRAGG WHAT IS A CANINE BREED? What is a breed? To put the question more precisely, what are the necessary conditions that enable us to say with conviction, "this group of animals constitutes a distinct breed?" In the cynological world, three separate approaches combine to constitute canine breeds. Dogs are distinguished first by ancestry , all of the individuals descending from a particular founder group (and only from that group) being designated as a breed. Next they are distinguished by purpose or utility, some breeds existing for the purpose of hunting particular kinds of game,others for the performance of particular tasks in cooperation with their human masters, while yet others owe their existence simply to humankind's desire for animal companionship. Finally dogs are distinguished by typology , breed standards (whether written or unwritten) being used to describe and to recognize dogs of specific size, physical build, general appearance, shape of head, style of ears and tail, etc., which are said to be of the same breed owing to their similarity in the foregoing respects. The preceding statements are both obvious and known to all breeders and fanciers of the canine species. Nevertheless a correct and full understanding of these simple truisms is vital to the proper functioning of the entire canine fancy and to the health and well being of the animals which are the object of that fancy. It is my purpose in this brief to elucidate the interrelationship of the above three approaches, to demonstrate how distortions and misunderstandings of that interrelationship now threaten the health of all of our dogs and the very existence of the various canine breeds, and to propose reforms which will restore both balanced breed identity and genetic health to CKC breeds. -

Pedigree Analysis and Optimisation of the Breeding Programme of the Markiesje and the Stabyhoun
Pedigree analysis and optimisation of the breeding programme of the Markiesje and the Stabyhoun Aiming to improve health and welfare and maintain genetic diversity Harmen Doekes REG.NR.: 920809186050 Major thesis Animal Breeding and Genetics (ABG-80436) January, 2016 Supervisors/examiners: Piter Bijma - Animal Breeding and Genomics Centre, Wageningen University Kor Oldenbroek - Centre for Genetic Resources the Netherlands (CGN), Wageningen UR Jack Windig - Animal Breeding & Genomics Centre, Wageningen UR Livestock Research Thesis: Animal Breeding and Genomics Centre Pedigree analysis and optimisation of the breeding programme of the Markiesje and the Stabyhoun Aiming to improve health and welfare and maintain genetic diversity Harmen Doekes REG.NR.: 920809186050 Major thesis Animal Breeding and Genetics (ABG-80436) January, 2016 Supervisors: Kor Oldenbroek - Centre for Genetic Resources the Netherlands (CGN), Wageningen UR Jack Windig - Animal Breeding & Genomics Centre, Wageningen UR Livestock Research Examiners: Piter Bijma - Animal Breeding and Genomics Centre, Wageningen University Kor Oldenbroek - Centre for Genetic Resources the Netherlands (CGN), Wageningen UR Commissioned by: Nederlandse Markiesjes Vereniging Nederlandse Vereniging voor Stabij- en Wetterhounen Preface This major thesis is submitted in partial fulfilment of the requirements for the degree of Master of Animal Sciences of Wageningen University, the Netherlands. It comprises an unpublished study on the genetic status of two Dutch dog breeds, the Markiesje and the Stabyhoun. that was commissioned by the Breed Clubs of the breeds, the ‘Nederlandse Markiesjes Vereniging’ and the ‘Nederlandse Vereniging voor Stabij- en Wetterhounen’. It was written for readers with limited pre-knowledge. Although the thesis focusses on two breeds, it addresses issues that are found in many dog breeds. -

The Ghost of Outcrossing Past in Downy Brome, an Inbreeding Annual Grass
Journal of Heredity 2013:104(4):476–490 Published by Oxford University Press on behalf of The American Genetic doi:10.1093/jhered/est019 Association 2013. This work is written by (a) US Government employee(s) Advance Access publication April 5, 2013 and is in the public domain in the US. The Ghost of Outcrossing Past in Downy Brome, an Inbreeding Annual Grass SUSAN E. MEYER, SUDEEP GHIMIRE, SAMUEL DECKER, KEITH R. MERRILL, AND CRAIG E. COLEMAN From the Shrub Sciences Laboratory, US Forest Service, Rocky Mountain Research Station, 735 North 500 East, Provo, UT 84606 (Meyer); Department of Plant and Wildlife Sciences, Brigham Young University, Provo, UT (Ghimire, Decker, and Coleman); and Center for Plant Breeding and Applied Plant Genomics, North Carolina State University, Raleigh, NC (Merrill). Address correspondence to Susan Meyer at the address above, or e-mail: [email protected] Abstract We investigated the frequency of outcrossing in downy brome (Bromus tectorum L.), a cleistogamous weedy annual grass, in both common garden and wild populations, using microsatellite and single nucleotide polymorphic (SNP) markers. In the common garden study, 25 lines with strongly contrasting genotypes were planted in close proximity. We fingerprinted 10 seed progeny from 8 individuals of each line and detected 15 first-generation heterozygotes for a t-value (corrected for cryptic crosses) of 0.0082. Different genotypes were significantly overrepresented as maternal versus paternal parents of heterozy- gotes, suggesting gender–function-dependent genetic control of outcrossing rates. In 4 wild populations (>300 individuals each), expected heterozygosity ranged from 0.149 to 0.336, whereas t-values ranged from 0.0027 to 0.0133, indicating high levels of both genetic diversity and inbreeding. -

Sex in the Extremes: Lichen-Forming Fungi
Mycologist, Volume 19, Part 2 May 2005. ©Cambridge University Press Printed in the United Kingdom. DOI: 10.1017/S0269915XO5002016 Sex in the extremes: lichen-forming fungi FABIAN A. SEYMOUR, PETER D. CRITTENDEN & PAUL S. DYER* School of Biology, University of Nottingham, University Park, Nottingham, NG7 2RD, UK. Tel. +44 (0) 115 9513203, Fax +44 (0) 115 9513251 E-mail: [email protected]; [email protected] *Corresponding Author Lichens are characteristically found in environments subject to extremes of temperature, desiccation and low nutrient status. Despite this sexual structures are often formed in abundance. The underlying mechanisms of sex in lichen-forming fungi are discussed, together with possible ecological reasons for the persistence of sexuality. Special features of lichen sex are highlighted including sex at the limits of life on earth in Antarctica, re-licheniza- tion following sex and dispersal, and the perennial nature of lichen fruiting bodies. Keywords: lichen, fungi, sex, breeding system, (98%) belonging to the Ascomycotina (Kirk et al., symbiosis, extreme environments, Antarctica 2001). They display a variety of morphologies, from flattened crust (crustose) or leafy (foliose) forms to Lichens - living together in a long-term relation- shrubby or pendulous fruticose types (Honegger, 2001) ship (Figs 3, 4, 7, 8). Lichens are seen as a textbook example of a successful Life in extreme environments mutualistic symbiosis. They consist of at least two A key characteristic of lichens is that they have a organisms: a fungus (the ‘mycobiont’), and an remarkable ability to tolerate extreme environmental intimately associated photosynthetic partner (the conditions and sustain growth despite frequent cycles ‘photobiont’). -

<I>Steinernema Carpocapsae
University of Nebraska - Lincoln DigitalCommons@University of Nebraska - Lincoln U.S. Department of Agriculture: Agricultural Publications from USDA-ARS / UNL Faculty Research Service, Lincoln, Nebraska 2011 Outcrossing and crossbreeding recovers deteriorated traits in laboratory cultured Steinernema carpocapsae nematodes John M. Chaston Brigham Young University Adler R. Dillman Brigham Young University David I. Shapiro-Ilan USDA-ARS Anwar L. Bilgrami Rutgers University Randy Gaugler Rutgers University See next page for additional authors Follow this and additional works at: https://digitalcommons.unl.edu/usdaarsfacpub Part of the Agricultural Science Commons Chaston, John M.; Dillman, Adler R.; Shapiro-Ilan, David I.; Bilgrami, Anwar L.; Gaugler, Randy; Hopper, Keith R.; and Adams, Byron J., "Outcrossing and crossbreeding recovers deteriorated traits in laboratory cultured Steinernema carpocapsae nematodes" (2011). Publications from USDA-ARS / UNL Faculty. 842. https://digitalcommons.unl.edu/usdaarsfacpub/842 This Article is brought to you for free and open access by the U.S. Department of Agriculture: Agricultural Research Service, Lincoln, Nebraska at DigitalCommons@University of Nebraska - Lincoln. It has been accepted for inclusion in Publications from USDA-ARS / UNL Faculty by an authorized administrator of DigitalCommons@University of Nebraska - Lincoln. Authors John M. Chaston, Adler R. Dillman, David I. Shapiro-Ilan, Anwar L. Bilgrami, Randy Gaugler, Keith R. Hopper, and Byron J. Adams This article is available at DigitalCommons@University of Nebraska - Lincoln: https://digitalcommons.unl.edu/ usdaarsfacpub/842 International Journal for Parasitology 41 (2011) 801–809 Contents lists available at ScienceDirect International Journal for Parasitology journal homepage: www.elsevier.com/locate/ijpara Outcrossing and crossbreeding recovers deteriorated traits in laboratory cultured Steinernema carpocapsae nematodes John M. -

Let's Talk Linebreeding
Course #304 Dog Breeding Basenji University “Preserving Our Past and Educating Our Future” Let’s Talk Linebreeding Claudia Waller Orlandi, Ph.D. Claudia Waller Orlandi, Ph.D. has been in dogs for 40 years and is well known for her Topsfield Bassets. She was 2009 AKC Breeder of the Year. She has a background in education. She writes and lectures frequently. Copyright by the author From Tally Ho. July-August 1997 One of the most bandied about terms among Basset Hound breeders today seems to be linebreeding. Despite its widespread use, however, linebreeding is frequently misunderstood and miscommunicated; in fact, it is not altogether uncommon for an outcrossed pedigree to be mistakenly viewed as linebreeding by the novice. The present discussion defines linebreeding and how we can more accurately describe our linebred litters. LINEBREEDING AND INBREEDING: A FAMILY AFFAIR INBREEDING and LINEBREEDING involve the mating of animals within the same family. Breeding relatives is used to cement traits, the goal being to make the offspring homozygous (pure) for desirable characteristics. Homozygous dogs tend to be prepotent and produce offspring that look like themselves (Walkowicz & Wilcox 1994). Willis (1989) defines INBREEDING as the mating of animals "more closely related to one another than the average relationship within the breed." Inbred pairings would include brother/sister (the closest form), father/daughter, mother/son and half-brother/half-sister. LINEBREEDING involves breeding relatives other than the individual parents or brothers and sisters. Typical linebred matings are grandfather/granddaughter, grandmother/grandson, grandson/granddaughter, great-granddaughter/great-grandson, uncle/niece, aunt/nephew and cousin crosses. -
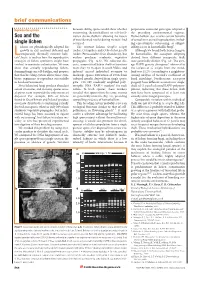
Brief Comms Layout 6/4Mx
brief communications Reproductive systems between sibling spores would show whether perpetuates successful genotypes adapted to outcrossing (heterothallism) or self-fertil- the prevailing environmental regimes. Sex and the ization (homothallism: allowing the fusion Homothallism also retains certain benefits of two identical nuclei during meiosis) had of sexual over asexual reproduction, includ- single lichen occurred. ing opportunistic outcrossing, as obligate ichens are physiologically adapted for The crustose lichens Graphis scripta selfing is rare in homothallic fungi8. growth in dry, nutrient-deficient and (order: Ostropales) and Ochrolechia parella Although we found both lichen fungi to Ltemporarily thermally extreme habi- (order: Pertusariales) fruit abundantly, but be homothallic, the ascospore offspring tats1, but it is unclear how the reproductive neither produces symbiotic vegetative derived from different conspecific thalli strategies of lichen symbionts might have propagules (Fig. 1a,b). We collected dis- were genetically distinct (Fig. 1d). The aver- evolved to maximize colonization. We now crete, symmetrical lichen thalli at locations age RAPD genetic divergence9 observed in show that sexually reproducing lichen- more than 10 m apart in south Wales, and five isolates of G. scripta from one wood- forming fungi can self-fertilize, and propose induced excised individual ascomata to land was 15.2% (according to a neighbour- that this breeding system allows these sym- discharge spores. Extraction of DNA from joining analysis of Jaccard’s coefficient of biotic organisms to reproduce successfully cultured mycelia derived from single spores band matching). Furthermore, ascospore in harsh environments. gave 218–263 randomly amplified poly- progeny from different ascomata on ‘single’ Most lichenized fungi produce abundant morphic DNA (RAPD) markers4 for each thalli of O. -

Heterosis from Local Drift Load Is Likely Insufficient to Favor Reversions To
bioRxiv preprint doi: https://doi.org/10.1101/273946; this version posted February 28, 2018. The copyright holder for this preprint (which was not certified by peer review) is the author/funder, who has granted bioRxiv a license to display the preprint in perpetuity. It is made available under aCC-BY-NC-ND 4.0 International license. Heterosis from local drift load is likely insufficient to favor reversions to outcrossing Abstract 2 The evolutionary trajectory from cross- to self-fertilization is widely documented in nature, but re- sults from several taxa also suggest that outcrossing may evolve in a formerly selfing population. Pop- 4 ulation genetic theory explains that selfing can evolve when its advantages overcome its immediate cost of inbreeding depression, but that this process will not run in reverse because a self-fertilizing population 6 purges itself of inbreeding depression. That is, the primary short-term advantage of cross-fertilization over self-fertilization depends on the existence of deleterious alleles exposed upon inbreeding. Here, 8 we explore whether outcrossing can evolve in selfing populations if allelic variation exists as divergence among populations. We consider two monomorphic populations of entirely self-fertilizing individuals, 10 introduce a modifier allele that increases the rate of cross-fertilization, and investigate whether the het- erosis among populations is sufficient for the modifier to invade and fix. We find that, despite an initial 12 increase in the frequency of the outcrossing modifier, its fixation is possible only when populations har- bor extremely large unique fixed genetic loads. These rare reversions to outcrossing become more likely 14 as the load becomes more polygenic, or when the modifier appears on a rare background, such as by dispersal of an outcrossing genotype into a selfing population. -

A Model for the Estimation of Outcrossing
Heredity (1981),47(1),35-52 0018-067X/81/03150035$02.OO 1981. The Genetical Society of Great Britain AMODEL FOR THE ESTIMATION OF OUTCROSSING RATE AND GENE FREQUENCIES USING n INDEPENDENT LOCI KERMIT RITLAND and SUBODH JAIN Department ofAgronomyand Range Science, University of California, Davis, CA 95616 Received19.vi.80 SUMMARY A mixed mating model for many unlinked loci is described. A procedure for estimation of the model parameters (outcrossing rate and gene frequencies), based on a multilocus maximum likelihood equation, is discussed and analyzed for bias, variance, and robustness. Genotypic data from families of known or unknown maternal parentage, or data from progenies of known maternal paren- tage, are used for estimation. The procedure is applicable to dominant or co-dominant Mendelian genes with two or three alleles per locus, and should be particularly useful in studies where the effort in scoring more loci is less than the effort in scoring more progeny. Variances of the multilocus estimates of outcrossing rate and pollen pool gene frequencies decrease when more loci are included in the estimation. Monte Carlo simulations showed the estimates to be unbiased when model assumptions are not violated, but the bias introduced by various violations is reduced when more loci are included in the estimate. Often the variance of a three or four locus estimate closely approaches the minimum variance possible (the variance of an estimate using infinitely many loci), setting a practical limit to the number of loci needed for a nearly minimum variance estimate. An example from some work on Limnanthes is presented to illustrate the use of multilocus model and its fit to data from natural popula- tions. -

The Classification of Lower Organisms
The Classification of Lower Organisms Ernst Hkinrich Haickei, in 1874 From Rolschc (1906). By permission of Macrae Smith Company. C f3 The Classification of LOWER ORGANISMS By HERBERT FAULKNER COPELAND \ PACIFIC ^.,^,kfi^..^ BOOKS PALO ALTO, CALIFORNIA Copyright 1956 by Herbert F. Copeland Library of Congress Catalog Card Number 56-7944 Published by PACIFIC BOOKS Palo Alto, California Printed and bound in the United States of America CONTENTS Chapter Page I. Introduction 1 II. An Essay on Nomenclature 6 III. Kingdom Mychota 12 Phylum Archezoa 17 Class 1. Schizophyta 18 Order 1. Schizosporea 18 Order 2. Actinomycetalea 24 Order 3. Caulobacterialea 25 Class 2. Myxoschizomycetes 27 Order 1. Myxobactralea 27 Order 2. Spirochaetalea 28 Class 3. Archiplastidea 29 Order 1. Rhodobacteria 31 Order 2. Sphaerotilalea 33 Order 3. Coccogonea 33 Order 4. Gloiophycea 33 IV. Kingdom Protoctista 37 V. Phylum Rhodophyta 40 Class 1. Bangialea 41 Order Bangiacea 41 Class 2. Heterocarpea 44 Order 1. Cryptospermea 47 Order 2. Sphaerococcoidea 47 Order 3. Gelidialea 49 Order 4. Furccllariea 50 Order 5. Coeloblastea 51 Order 6. Floridea 51 VI. Phylum Phaeophyta 53 Class 1. Heterokonta 55 Order 1. Ochromonadalea 57 Order 2. Silicoflagellata 61 Order 3. Vaucheriacea 63 Order 4. Choanoflagellata 67 Order 5. Hyphochytrialea 69 Class 2. Bacillariacea 69 Order 1. Disciformia 73 Order 2. Diatomea 74 Class 3. Oomycetes 76 Order 1. Saprolegnina 77 Order 2. Peronosporina 80 Order 3. Lagenidialea 81 Class 4. Melanophycea 82 Order 1 . Phaeozoosporea 86 Order 2. Sphacelarialea 86 Order 3. Dictyotea 86 Order 4. Sporochnoidea 87 V ly Chapter Page Orders. Cutlerialea 88 Order 6.