Oxygen, Life Forms, and the Evolution of Sexes in Multicellular Eukaryotes
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Evolution of Genetic Systems in Filamentous Ascomycetes
Evolution of Genetic Systems in Filamentous Ascomycetes Evolutie van genetische systemen in hyphenvormende zakjeszwammen 0000 0513 3836 Promotor: dr. R.F. Hoekstra hoogleraar in de populatie- en kwantitatieve genetica fjtfoiißi f ßin Maarten J. Nauta Evolution of Genetic Systems in Filamentous Ascomycetes Proefschrift ter verkrijging van de graad van doctor in de landbouw- en milieuwetenschappen op gezag van de rector magnificus, dr. C.M. Karssen, in het openbaar te verdedigen op woensdag 12januar i 1994 des namiddags te vier uur in de Aula van de Landbouwuniversiteit te Wageningen. 15 0 S(p^ZJ> These investigations were supported by the Netherlands Organization for Scientific Research (N.W.O.). BibUt/FHEEK LAMDbOirWUNIVERSITEJi. WAGE NINGE N CIP-GEGEVENS KONINKLIJKE BIBLIOTHEEK, DEN HAAG Nauta, Maarten J. Evolution of genetic systems in filamentous ascomycetes / Maarten J. Nauta. - [ S.l. : s.n.]. -111 . Thesis Wageningen. - With ref. - With summary in Dutch. ISBN 90-5485-199-6 Subject headings: population genetics / ascomycetes. omslagontwerp: Ernst van Cleef foto omslag: Barrages tussen verschillende stammen van Podospora anserina als gevolg van vegetatieve incompatibiliteit. (met dank aan Inge Haspels) aan mijn ouders Voorwoord Dit proefschrift is het resultaat van vier jaar onderzoek, verricht bij de vakgroep Erfelijkheidsleer van de Landbouwuniversiteit in Wageningen. In zekere zin valt zo'n proefschrift te vergelijken met een levend wezen. Uit de genetica is bekend dat de verschijningsvorm van elk levend wezen tot stand komt door een combinatie van erfelijke aanleg en invloeden uit de omgeving. Voor een proefschrift geldt eigenlijk hetzelfde: Zowel het werk van de auteur, als de bijdragen van zijn omgeving zijn onontbeerlijk om tot een verschijningsvorm te komen. -

Alternative Oxidase: a Mitochondrial Respiratory Pathway to Maintain Metabolic and Signaling Homeostasis During Abiotic and Biotic Stress in Plants
Int. J. Mol. Sci. 2013, 14, 6805-6847; doi:10.3390/ijms14046805 OPEN ACCESS International Journal of Molecular Sciences ISSN 1422-0067 www.mdpi.com/journal/ijms Review Alternative Oxidase: A Mitochondrial Respiratory Pathway to Maintain Metabolic and Signaling Homeostasis during Abiotic and Biotic Stress in Plants Greg C. Vanlerberghe Department of Biological Sciences and Department of Cell and Systems Biology, University of Toronto Scarborough, 1265 Military Trail, Toronto, ON, M1C1A4, Canada; E-Mail: [email protected]; Tel.: +1-416-208-2742; Fax: +1-416-287-7676 Received: 16 February 2013; in revised form: 8 March 2013 / Accepted: 12 March 2013 / Published: 26 March 2013 Abstract: Alternative oxidase (AOX) is a non-energy conserving terminal oxidase in the plant mitochondrial electron transport chain. While respiratory carbon oxidation pathways, electron transport, and ATP turnover are tightly coupled processes, AOX provides a means to relax this coupling, thus providing a degree of metabolic homeostasis to carbon and energy metabolism. Beside their role in primary metabolism, plant mitochondria also act as “signaling organelles”, able to influence processes such as nuclear gene expression. AOX activity can control the level of potential mitochondrial signaling molecules such as superoxide, nitric oxide and important redox couples. In this way, AOX also provides a degree of signaling homeostasis to the organelle. Evidence suggests that AOX function in metabolic and signaling homeostasis is particularly important during stress. These include abiotic stresses such as low temperature, drought, and nutrient deficiency, as well as biotic stresses such as bacterial infection. This review provides an introduction to the genetic and biochemical control of AOX respiration, as well as providing generalized examples of how AOX activity can provide metabolic and signaling homeostasis. -

Rhetoric and Plants Alana Hatley University of South Carolina
University of South Carolina Scholar Commons Theses and Dissertations 2018 Rhetoric and Plants Alana Hatley University of South Carolina Follow this and additional works at: https://scholarcommons.sc.edu/etd Part of the English Language and Literature Commons Recommended Citation Hatley, A.(2018). Rhetoric and Plants. (Doctoral dissertation). Retrieved from https://scholarcommons.sc.edu/etd/4858 This Open Access Dissertation is brought to you by Scholar Commons. It has been accepted for inclusion in Theses and Dissertations by an authorized administrator of Scholar Commons. For more information, please contact [email protected]. Rhetoric and Plants by Alana Hatley Bachelor of Arts Northeastern State University, 2006 Master of Arts Northeastern State University, 2010 Submitted in Partial Fulfillment of the Requirements For the Degree of Doctor of Philosophy in English College of Arts and Sciences University of South Carolina 2018 Accepted by: John Muckelbauer, Major Professor Mindy Fenske, Committee Member Byron Hawk, Committee Member Jeffrey T. Nealon, Committee Member Cheryl L. Addy, Vice Provost and Dean of the Graduate School © Copyright by Alana Hatley, 2018 All Rights Reserved. ii Acknowledgements So many people. Thank you to the First-Year English department at the University of South Carolina for giving me the opportunity to support myself while doing work that I truly care about. Similar thanks are due to the faculty at Northeastern State University, without whom I would never have arrived at USC. I also want to thank not only my teachers but also my students; your thoughts and minds have influenced mine in ways I cannot articulate. Thank you to Lisa Bailey, Erica Fischer, Amber Lee, Trevor C. -

Algal Sex Determination and the Evolution of Anisogamy James Umen, Susana Coelho
Algal Sex Determination and the Evolution of Anisogamy James Umen, Susana Coelho To cite this version: James Umen, Susana Coelho. Algal Sex Determination and the Evolution of Anisogamy. Annual Review of Microbiology, Annual Reviews, 2019, 73 (1), 10.1146/annurev-micro-020518-120011. hal- 02187088 HAL Id: hal-02187088 https://hal.sorbonne-universite.fr/hal-02187088 Submitted on 17 Jul 2019 HAL is a multi-disciplinary open access L’archive ouverte pluridisciplinaire HAL, est archive for the deposit and dissemination of sci- destinée au dépôt et à la diffusion de documents entific research documents, whether they are pub- scientifiques de niveau recherche, publiés ou non, lished or not. The documents may come from émanant des établissements d’enseignement et de teaching and research institutions in France or recherche français ou étrangers, des laboratoires abroad, or from public or private research centers. publics ou privés. Annu. Rev. Microbiol. 2019. 73:X–X https://doi.org/10.1146/annurev-micro-020518-120011 Copyright © 2019 by Annual Reviews. All rights reserved Umen • Coelho www.annualreviews.org • Algal Sexes and Mating Systems Algal Sex Determination and the Evolution of Anisogamy James Umen1 and Susana Coelho2 1Donald Danforth Plant Science Center, St. Louis, Missouri 63132, USA; email: [email protected] 2Sorbonne Université, UPMC Université Paris 06, CNRS, Algal Genetics Group, UMR 8227, Integrative Biology of Marine Models, Station Biologique de Roscoff, CS 90074, F-29688, Roscoff, France [**AU: Please write the entire affiliation in French or write it all in English, rather than a combination of English and French**] ; email: [email protected] Abstract Algae are photosynthetic eukaryotes whose taxonomic breadth covers a range of life histories, degrees of cellular and developmental complexity, and diverse patterns of sexual reproduction. -

Sex in the Extremes: Lichen-Forming Fungi
Mycologist, Volume 19, Part 2 May 2005. ©Cambridge University Press Printed in the United Kingdom. DOI: 10.1017/S0269915XO5002016 Sex in the extremes: lichen-forming fungi FABIAN A. SEYMOUR, PETER D. CRITTENDEN & PAUL S. DYER* School of Biology, University of Nottingham, University Park, Nottingham, NG7 2RD, UK. Tel. +44 (0) 115 9513203, Fax +44 (0) 115 9513251 E-mail: [email protected]; [email protected] *Corresponding Author Lichens are characteristically found in environments subject to extremes of temperature, desiccation and low nutrient status. Despite this sexual structures are often formed in abundance. The underlying mechanisms of sex in lichen-forming fungi are discussed, together with possible ecological reasons for the persistence of sexuality. Special features of lichen sex are highlighted including sex at the limits of life on earth in Antarctica, re-licheniza- tion following sex and dispersal, and the perennial nature of lichen fruiting bodies. Keywords: lichen, fungi, sex, breeding system, (98%) belonging to the Ascomycotina (Kirk et al., symbiosis, extreme environments, Antarctica 2001). They display a variety of morphologies, from flattened crust (crustose) or leafy (foliose) forms to Lichens - living together in a long-term relation- shrubby or pendulous fruticose types (Honegger, 2001) ship (Figs 3, 4, 7, 8). Lichens are seen as a textbook example of a successful Life in extreme environments mutualistic symbiosis. They consist of at least two A key characteristic of lichens is that they have a organisms: a fungus (the ‘mycobiont’), and an remarkable ability to tolerate extreme environmental intimately associated photosynthetic partner (the conditions and sustain growth despite frequent cycles ‘photobiont’). -

Origin of Chordates Part-I
ORIGIN OF CHORDATES Evolution of chordate was one of the most important event in the history of chordate as it was the beginning of evolution of more advanced chordates like bird and mammals. It is the story of origin from a primitive invertebrate like creatures to early chordates. Though first fossil of the first vertebrate the ostrachoderm was discovered from the Ordovician period but it might have originated in late Cambrian period. As early chordates were soft bodied, their fossil records are not preserved. Hence, to trace their ancestry, we have to find out the similarity among different deuterostomes to trace the origin of chordates. Some structural features shared by them such as bilateral symmetry, antero-posterior body axis, triploblastic coelomate condition, etc., may he because of their common ancestry. TIME OF ORIGIN: Late Cambrian period PLACE OF ORIGIN: Fresh water THEORIES OF INVERTEBRATE ANCESTRY OF CHORDATES Several theories have been put forwarded to explain the origin of chordates either directly from some invertebrate group or through the intervention of some protochordate. Almost every invertebrate phylum—Coelenterata, Nemertean, Phoronida, Annelids, Arthropods and Echinodermatashas been suggested. But these theories are far from being satisfactory and convincing and have only a historical value. Only the echinoderm theory has received some acceptance. DIVISION OF BILATERIA The Bilateria is divided into two major divisions (1) Protostomia and (2) Deuterostornia. This division is based on the differences in embryonic and larval developments. Protostomia includes from Annelida to Arthropoda while deuterostomia includes Echinodermata, Pogonophora and Chordate. DEUTEROSTOME LINE OF CHORDATE EVOLUTION Following common features of all Deuterostomes suggests strong evidence of a closer evolutionary relationship between the three principal deuterostome phyla – Echinodermata, Hemichordata and Chordata. -
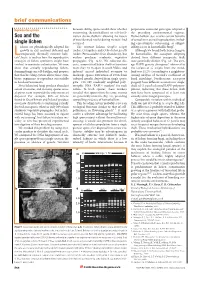
Brief Comms Layout 6/4Mx
brief communications Reproductive systems between sibling spores would show whether perpetuates successful genotypes adapted to outcrossing (heterothallism) or self-fertil- the prevailing environmental regimes. Sex and the ization (homothallism: allowing the fusion Homothallism also retains certain benefits of two identical nuclei during meiosis) had of sexual over asexual reproduction, includ- single lichen occurred. ing opportunistic outcrossing, as obligate ichens are physiologically adapted for The crustose lichens Graphis scripta selfing is rare in homothallic fungi8. growth in dry, nutrient-deficient and (order: Ostropales) and Ochrolechia parella Although we found both lichen fungi to Ltemporarily thermally extreme habi- (order: Pertusariales) fruit abundantly, but be homothallic, the ascospore offspring tats1, but it is unclear how the reproductive neither produces symbiotic vegetative derived from different conspecific thalli strategies of lichen symbionts might have propagules (Fig. 1a,b). We collected dis- were genetically distinct (Fig. 1d). The aver- evolved to maximize colonization. We now crete, symmetrical lichen thalli at locations age RAPD genetic divergence9 observed in show that sexually reproducing lichen- more than 10 m apart in south Wales, and five isolates of G. scripta from one wood- forming fungi can self-fertilize, and propose induced excised individual ascomata to land was 15.2% (according to a neighbour- that this breeding system allows these sym- discharge spores. Extraction of DNA from joining analysis of Jaccard’s coefficient of biotic organisms to reproduce successfully cultured mycelia derived from single spores band matching). Furthermore, ascospore in harsh environments. gave 218–263 randomly amplified poly- progeny from different ascomata on ‘single’ Most lichenized fungi produce abundant morphic DNA (RAPD) markers4 for each thalli of O. -

Melatonin Induces Apoptosis in Cholangiocarcinoma Cell Lines by Activating the Reactive Oxygen Species-Mediated Mitochondrial Pathway
ONCOLOGY REPORTS 33: 1443-1449, 2015 Melatonin induces apoptosis in cholangiocarcinoma cell lines by activating the reactive oxygen species-mediated mitochondrial pathway Umawadee LAOTHONG1-3,5, YUSUKE HIRAKU2, SHINJI Oikawa2, KITTI INTUYOD3,4, MARIKO Murata2* and SOMCHAI PINLAOR1,3* 1Department of Parasitology, Faculty of Medicine, Khon Kaen University, Khon Kaen 40002, Thailand; 2Department of Environmental and Molecular Medicine, Mie University Graduate School of Medicine, Mie 514-8507, Japan; 3Liver Fluke and Cholangiocarcinoma Research Center, Faculty of Medicine, Khon Kaen University, Khon Kaen 40002, Thailand; 4Biomedical Science Program, Graduate School, Khon Kaen University, Khon Kaen 40002, Thailand Received November 26, 2014; Accepted January 2, 2015 DOI: 10.3892/or.2015.3738 Abstract. We previously demonstrated that melatonin could pro-oxidant by activating ROS-dependent DNA damage and be used as a chemopreventive agent for inhibiting cholangio- thus leading to the apoptosis of CCA cells. carcinoma (CCA) development in a hamster model. However, the cytotoxic activity of melatonin in cancer remains unclear. Introduction In the present study, we investigated the effect of melatonin on CCA cell lines. Human CCA cell lines (KKU-M055 and Cholangiocarcinoma (CCA) is a devastating biliary cancer that KKU-M214) were treated with melatonin at concentrations poses continuing diagnostic and therapeutic challenges (1). of 0.5, 1 and 2 mM for 48 h. Melatonin treatment exerted a There are several risk factors for CCA: mainly liver fluke cytotoxic effect on CCA cells by inhibiting CCA cell viability infection, primary sclerosing cholangitis, biliary-duct cysts in a concentration-dependent manner. Treatment with mela- and hepatolithiasis (2). The highest prevalence of liver fluke tonin, especially at 2 mM, increased intracellular reactive Opisthorchis viverrini infection has been reported in Northeast oxygen species (ROS) production and in turn led to increased Thailand, where CCA incidence is also high (3). -

Padgettia, a New Ge~Us Based on Fertile Ieurop Teroid
PADGETTIA, A NEW GE~US BASED ON FERTILE IEUROP TEROID FOLIAGE FROM THE PEH.MIAN OF TEX \S* SERGIUS H. MAi:vIAY .S. Geological Survey, \>Vashington 25, D.C. s Permian floras become increasingly to several adnate, sef'd-like fructifications, well-known, it becomes more apparent probably imbedded in the lZLIllina, aligned A that the Permian period produced parallel to the venation on either side of the many peculiar plant forms that defy close midrib. comparison with plants of the present. Pad Fronds at kast once pinnat ; pinnules gettia, the new genus to be described here, is apparently isophyllous, long (12 em. or morC') represen ted by one of these morphologically and relatively narrow (to 3 cm. wide) with anomalous types of plants. entire margins and cordate bases, the bminae The material upon which this account is tapering gently to acut apices. Pinnule based was found in an assemblage of plant midribs to 3 mm. wide, finely --triated; lateral compressions collected in 1939 by Charles veins clo ely spac d, departing decurrently B. Read, U.S. Geological Survey, at a locality at an angle of approximately 30 degrees, " approximately 2 miles northwest of Pad arching to meet pinnule margins nearly gett ", in Young County, Texas, (U.s.G.S. perpendicularly, usually forking once within Palaeobotanical Locality Number 8967). a short distance of the midrib According to the geological map of Young Fructifications ovoid, elliptical or pyriform, County (Plummer and Fuqua, 1937), out from 2·5 to 9·0 mm. long and 2·0 to 5·0 mm. crops in that area lie within the Lower wide, usually borne one or two, occasionally Permian Moran formation of the Wichita several to a pinnule, being evel Iy . -

Base Excision Repair Synthesis of DNA Containing 8-Oxoguanine in Escherichia Coli
EXPERIMENTAL and MOLECULAR MEDICINE, Vol. 35, No. 2, 106-112, April 2003 Base excision repair synthesis of DNA containing 8-oxoguanine in Escherichia coli Yun-Song Lee1,3 and Myung-Hee Chung2 Introduction 1Division of Pharmacology 8-oxo-7,8-dihydroguanine (8-oxo-G) in DNA is a muta- Department of Molecular and Cellular Biology genic adduct formed by reactive oxygen species Sungkyunkwan University School of Medicine (Kasai and Nishimura, 1984). As a structural prefe- Suwon 440-746, Korea rence, adenine is frequently incorporated into oppo- 2Department of Pharmacology site template 8-oxo-G (Shibutani et al., 1991), and 8- Seoul National University College of Medicine oxo-dGTP is incorporated into opposite template dA Jongno-gu, Seoul 110-799, Korea during DNA synthesis (Cheng et al., 1992). Thus, un- 3Corresponding author: Tel, 82-31-299-6190; repaired, these mismatches lead to GT and AC trans- Fax, 82-31-299-6209; E-mail, [email protected] versions, respectively (Grollman and Morya, 1993). In Escherichia coli, several DNA repair enzymes, Accepted 29 March 2003 preventing mutagenesis by 8-oxo-G, are known as the GO system (Michaels et al., 1992). The GO system Abbreviations: 8-oxo-G, 8-oxo-7,8-dihydroguanine; Fapy, 2,6-dihy- consists of MutT (8-oxo-dGTPase), MutM (2,6-dihydro- droxy-5N-formamidopyrimidine; FPG, Fapy-DNA glycosylase; BER, xy-5N-formamidopyrimidine (Fapy)-DNA glycosylase, base excision repair; AP, apurinic/apyrimidinic; dRPase, deoxyribo- Fpg) and MutY (adenine-DNA glycosylase). 8-oxo- phosphatase GTPase prevents incorporation of 8-oxo-dGTP into DNA by degrading 8-oxo-dGTP. -

Reactive Oxygen Species from Chloroplasts Contribute to 3-Acetyl-5- Isopropyltetramic Acid-Induced Leaf Necrosis of Arabidopsis Thaliana
Plant Physiology and Biochemistry 52 (2012) 38e51 Contents lists available at SciVerse ScienceDirect Plant Physiology and Biochemistry journal homepage: www.elsevier.com/locate/plaphy Research article Reactive oxygen species from chloroplasts contribute to 3-acetyl-5- isopropyltetramic acid-induced leaf necrosis of Arabidopsis thaliana Shiguo Chen a,1, Chunyan Yin a,1, Reto Jörg Strasser a,b,c, Govindjee d,2, Chunlong Yang a, Sheng Qiang a,* a Weed Research Laboratory, Nanjing Agricultural University, Nanjing 210095, China b Bioenergetics Laboratory, University of Geneva, CH-1254 Jussy/Geneva, Switzerland c North West University of South Africa, South Africa d Department of Plant Biology, and Department of Biochemistry, University of Illinois at Urbana-Champaign, Urbana, IL 61801, USA article info abstract Article history: 3-Acetyl-5-isopropyltetramic acid (3-AIPTA), a derivate of tetramic acid, is responsible for brown leaf- Received 22 August 2011 spot disease in many plants and often kills seedlings of both mono- and dicotyledonous plants. To further Accepted 2 November 2011 elucidate the mode of action of 3-AIPTA, during 3-AIPTA-induced cell necrosis, a series of experiments Available online 11 November 2011 were performed to assess the role of reactive oxygen species (ROS) in this process. When Arabidopsis thaliana leaves were incubated with 3-AIPTA, photosystem II (PSII) electron transport beyond QA (the Keywords: primary plastoquinone acceptor of PSII) and the reduction of the end acceptors at the PSI acceptor side 3-AIPTA (3-acetyl-5-isopropyltetramic acid) were inhibited; this was followed by increase in charge recombination and electron leakage to O , ROS (reactive oxygen species) 2 Cell death resulting in chloroplast-derived oxidative burst. -

Geology of Saipan Mariana Islands Part 4
Geology of Saipan Mariana Islands Part 4. Submarine Topography and Shoal- Water Ecology GEOLOGICAL SURVEY PROFESSIONAL PAPER 280-K Geology of Saipan Mariana Islands Part 4. Submarine Topography and Shoal- Water Ecology By PRESTON E. CLOUD, Jr. GEOLOGICAL SURVEY PROFESSIONAL PAPER 280-K Description and interpretation of the submarine topography and of the sediments^ biotas^ and morphology of the reef complex adjacent to a geologically diverse tropical island UNITED STATES GOVERNMENT PRINTING OFFICE, WASHINGTON : 1959 UNITED STATES DEPARTMENT OF THE INTERIOR FRED A. S EATON, Secretary GEOLOGICAL SURVEY Thomas B. Nolan, Director For sale by the Superintendent of Documents, U. S. Government Printing Office Washington 25, D. C. GEOLOGICAL SURVEY PROFESSIONAL PAPER 280 Geology of Saipan, Mariana Islands Part 1. General Geology A. General Geology By PRESTON E. CLOUD, Jr., ROBERT GEORGE SCHMIDT, and HAROLD W. BURKE Part 2. Petrology and Soils B. Petrology of the Volcanic Rocks By ROBERT GEORGE SCHMIDT C. Petrography of the Limestones By J. HARLAN JOHNSON D. Soils By RALPH J. McCRACKEN Part 3. Paleontology E. Calcareous Algae By J. HARLAN JOHNSON F. Difcoaster and Some Related Microfossils By M. N. BRAMLETTE G. Eocene Radiolaria By WILLIAM RIEDEL H. Smaller Foraminifera By RUTH TODD I. Larger Foraminifera By W. STORRS COLE J. Echinoids By C. WYTHE COOKE Part 4. Submarine Topography and Shoal-Water Ecology K. Submarine Topography and Shoal-Water Ecology By PRESTON E. CLOUD, Jr. CONTENTS Page Page Abstract_________________________________________ 361 Shoal-water and shoreline ecology and sediments—Con. Introduction. ______________________________________ 362 Habitat descriptions—Con. Purpose and scope of the work_____________________ 362 Organic reefs and reef benches______________ 383 Field methods and acknowledgments-_______________ 362 Minor reef structures______________________ 384 Systematic identifications and other research aid____ 363 Biotope X.