Report to the Fish and Game Commission
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-
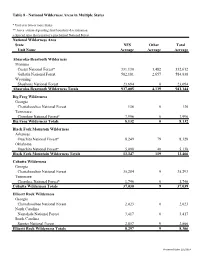
Table 8 — National Wilderness Areas in Multiple States
Table 8 - National Wilderness Areas in Multiple States * Unit is in two or more States ** Acres estimated pending final boundary determination + Special Area that is part of a proclaimed National Forest National Wilderness Area State NFS Other Total Unit Name Acreage Acreage Acreage Absaroka-Beartooth Wilderness Montana Custer National Forest* 331,130 1,482 332,612 Gallatin National Forest 582,181 2,657 584,838 Wyoming Shoshone National Forest 23,694 0 23,694 Absaroka-Beartooth Wilderness Totals 937,005 4,139 941,144 Big Frog Wilderness Georgia Chattahoochee National Forest 136 0 136 Tennessee Cherokee National Forest* 7,996 0 7,996 Big Frog Wilderness Totals 8,132 0 8,132 Black Fork Mountain Wilderness Arkansas Ouachita National Forest* 8,249 79 8,328 Oklahoma Ouachita National Forest* 5,098 40 5,138 Black Fork Mountain Wilderness Totals 13,347 119 13,466 Cohutta Wilderness Georgia Chattahoochee National Forest 35,284 9 35,293 Tennessee Cherokee National Forest* 1,746 0 1,746 Cohutta Wilderness Totals 37,030 9 37,039 Ellicott Rock Wilderness Georgia Chattahoochee National Forest 2,023 0 2,023 North Carolina Nantahala National Forest 3,417 0 3,417 South Carolina Sumter National Forest 2,857 9 2,866 Ellicott Rock Wilderness Totals 8,297 9 8,306 Processed Date: 2/5/2014 Table 8 - National Wilderness Areas in Multiple States * Unit is in two or more States ** Acres estimated pending final boundary determination + Special Area that is part of a proclaimed National Forest National Wilderness Area State NFS Other Total Unit Name Acreage Acreage -

Table 8 - National Wilderness Areas in Multiple States
Table 8 - National Wilderness Areas in Multiple States * Unit is in two or more States ** Acres estimated pending final boundary determination + Special Area that is part of a proclaimed National Forest National Wilderness Area State NFS Other Total Unit Name Acreage Acreage Acreage Absaroka-Beartooth Wilderness Montana Custer National Forest* 331,130 1,482 332,612 Gallatin National Forest 582,181 2,657 584,838 Wyoming Shoshone National Forest 23,694 0 23,694 Absaroka-Beartooth Wilderness Total 937,005 4,139 941,144 Big Frog Wilderness Georgia Chattahoochee National Forest 136 0 136 Tennessee Cherokee National Forest* 7,996 0 7,996 Big Frog Wilderness Total 8,132 0 8,132 Black Fork Mountain Wilderness Arkansas Ouachita National Forest* 8,249 79 8,328 Oklahoma Ouachita National Forest* 5,098 40 5,138 Black Fork Mountain Wilderness Total 13,347 119 13,466 Cohutta Wilderness Georgia Chattahoochee National Forest 35,284 9 35,293 Tennessee Cherokee National Forest* 1,746 0 1,746 Cohutta Wilderness Total 37,030 9 37,039 Ellicott Rock Wilderness Georgia Chattahoochee National Forest 2,023 0 2,023 North Carolina Nantahala National Forest 3,417 0 3,417 South Carolina Sumter National Forest 2,857 9 2,866 Ellicott Rock Wilderness Total 8,297 9 8,306 Refresh Date: 10/18/2014 Table 8 - National Wilderness Areas in Multiple States * Unit is in two or more States ** Acres estimated pending final boundary determination + Special Area that is part of a proclaimed National Forest National Wilderness Area State NFS Other Total Unit Name Acreage Acreage Acreage -

The Siskiyou Hiker 2020
WINTER 2020 THE SISKIYOU HIKER Outdoor news from the Siskiyou backcountry SPECIAL ISSUE: 2020 Stewardship Report Photo by: Trevor Meyer SEASON UPDATES ALL THE TRAILS CLEARED THIS YEAR LOOKING AHEAD CHECK OUT OUR Laina Rose, 2020 Crew Leader PLANS FOR 2021 LETTER FROM THE DIRECTOR Winter, 2020 Dear Friends, In this special issue of the Siskiyou Hiker, we’ve taken our annual stewardship report and wrapped it up into a periodical for your review. Like everyone, 2020 has been a tough year for us. But I hope this issue illustrates that this year was a challenge we were up for. We had to make big changes, including a hiring freeze on interns and seasonals. My staff, board, our volun- teers, and I all had to flex into what roles needed to be filled, and far-ahead planning became almost impossi- ble. But we were able to wrap up technical frontcountry projects in the spring, and finished work on the Briggs Creek Bridge and a long retaining wall on the multi-use Taylor Creek Trail. Then my staff planned for a smaller intern program that was stronger beyond measure. We put practices in place to keep everyone safe, and got through the year intact and in good health. This year we had a greater impact on the lives of the young people who serve on our Wilderness Conserva- tion Corps. They completed media projects and gained technical skills. Everyone pushed themselves and we took the first real steps in realizing greater diversity throughout our organization. And despite protocols in place to slow the spread of Covid-19, we actually grew our volunteer program. -

9691.Ch01.Pdf
© 2006 UC Regents Buy this book University of California Press, one of the most distinguished univer- sity presses in the United States, enriches lives around the world by advancing scholarship in the humanities, social sciences, and natural sciences. Its activities are supported by the UC Press Foundation and by philanthropic contributions from individuals and institutions. For more information, visit www.ucpress.edu. University of California Press Berkeley and Los Angeles, California University of California Press, Ltd. London, England © 2006 by The Regents of the University of California Library of Congress Cataloging-in-Publication Data Sawyer, John O., 1939– Northwest California : a natural history / John O. Sawyer. p. cm. Includes bibliographical references and index. ISBN 0-520-23286-0 (cloth : alk. paper) 1. Natural history—California, Northern I. Title. QH105.C2S29 2006 508.794—dc22 2005034485 Manufactured in the United States of America 15 14 13 12 11 10 09 08 07 06 10987654321 The paper used in this publication meets the minimum require- ments of ansi/niso z/39.48-1992 (r 1997) (Permanence of Paper).∞ The Klamath Land of Mountains and Canyons The Klamath Mountains are the home of one of the most exceptional temperate coniferous forest regions in the world. The area’s rich plant and animal life draws naturalists from all over the world. Outdoor enthusiasts enjoy its rugged mountains, its many lakes, its wildernesses, and its wild rivers. Geologists come here to refine the theory of plate tectonics. Yet, the Klamath Mountains are one of the least-known parts of the state. The region’s complex pattern of mountains and rivers creates a bewil- dering set of landscapes. -

Step 3 - Current Conditions
Step 3 - Current Conditions INTRODUCTION - This step describes the current occurs during summer thunderstorms. Winter range, distribution and condition of ecosystem precipitation occurs mainly as snow above 4,000 feet elements. It is organized by Issue as presented in elevation, and mainly as rain below that elevation. Step 2 and answers Key Questions identified for Fluctuation of the snow level occasionally results in each issue of this step. rain causing rapid snow melt. PRECIPITATION AQUATICS The precipitation record is characterized by two distinct climate trends (Table 3-1). These alternating periods of wet and dry conditions lasted for a few HILLSLOPE PROCESSES decades. The short duration of the record and irregular nature of climatic change preclude Key Question 1- What are the dominant forecasting of these periods. The drier periods are of hydrologic and erosional characteristics and approximately 40 inches average annual processes within these watersheds, including precipitation, the wetter are of approximately 60 impacts of the 1997 flood? inches. The Happy Camp record, along with longer records from Eureka and other stations in Landslides introduce large volumes of coarse northwestern California, indicate that the period 1870 sediment to streams during episodes of intense to 1910 was a wet time; 1911 to 1937 dry; 1938 to precipitation. This results in changes in the structure 1975 wet; 1976 to 1994 dry and 1995 to present wet. of stream channels and the quality of instream Intense precipitation of 1982-83 and 1997 are related habitat. Episodes of large amounts of sediment to strong El Niño effects. The probability of production are followed by about 10 years of rapid occurrence of rare, intense storms is higher during adjustment of channel geometry. -

Public Law 98-425 An
PUBLIC LAW 98-425-SEPT. 28, 1984 98 STAT. 1619 Public Law 98-425 98th Congress An Act Sept. 28, 1984 Entitled the "California Wilderness Act of 1984". [H.R. 1437] Be it enacted by the Senate and House of Representatives of the United States of America in Congress assembled, That this title may California Wilderness Act be cited as the "California Wilderness Act of 1984". of 1984. National TITLE I Wilderness Preservation System. DESIGNATION OF WILDERNESS National Forest System. SEC. 101. (a) In furtherance of the purposes of the Wilderness Act, National parks, the following lands, as generally depicted on maps, appropriately monuments, etc. referenced, dated July 1980 (except as otherwise dated) are hereby 16 USC 1131 designated as wilderness, and therefore, as components of the Na note. tional Wilderness Preservation System- (1)scertain lands in the Lassen National Forest, California,s which comprise approximately one thousand eight hundred acres, as generally depicted on a map entitled "Caribou Wilder ness Additions-Proposed", and which are hereby incorporated in, and which shall be deemed to be a part of the Caribou Wilderness as designated by Public Law 88-577; 16 USC 1131 (2)s certain lands in the Stanislaus and Toiyabe Nationals note. 16 USC 1132 Forests, California, which comprise approximately one hundred note. sixty thousand acres, as generally depicted on a map entitled "Carson-Iceberg Wilderness-Proposed", dated July 1984, and which shall be known as the Carson-Iceberg Wilderness: Pro vided, however, That the designation of the Carson-Iceberg Wil derness shall not preclude continued motorized access to those previously existing facilities which are directly related to per mitted livestock grazing activities in the Wolf Creek Drainage on the Toiyabe National Forest in the same manner and degree in which such access was occurring as of the date of enactment of this title; (3)scertain lands in the Shasta-Trinity National Forest, Cali 16 USC 1132 fornia, which comprise approximately seven thousand three note. -

News Release
U.S. Forest Service Pacific Southwest Region Klamath National Forest News Release 1711 South Main Street September 12, 2020 Yreka, CA 96097 Media Contact: Public Information Office 530-324-2528 www.facebook.com/KlamathNF InciWeb: https://inciweb.nwcg.gov/incident/7173/ Slater and Devil Fire Evening Update for September 12, 2020, 9 p.m. HAPPY CAMP, CALIFORNIA — On the north (Oregon) side of the Slater Fire, firefighting resources scouted opportunities to construct fireline to keep the wildfire out of populated areas. Dozer lines now surround the communities of Sunstar, Takilma, and Holland. Siskiyou Mountain Ranger District initial attack firefighting resources have begun to prepare the 1040 road in the event that the Devil Fire should work its way out of the Red Buttes Wilderness. Portions of the 1040 road were treated in 2017 during the Miller Complex. Along with increased humidity, the Miller Complex Fire scar should slow or stop fire spread towards the Applegate Valley. Along its eastern edge, the fire has crossed into Sucker Creek and is burning in the Swan Mountain area, but is still far from structures. A public meeting will be held tomorrow, September 13, 2020 at 2:30 p.m. Slater Fire North Area Virtual Community Meeting Sunday, September 13, 2020, 2:30 p.m. Facebook Live @SlaterAndDevilFireInformation https://www.facebook.com/SlaterAndDevilFireInformation As of this evening, the Slater Fire has not made it into Steve’s Fork; fire personnel scouted out the area several days ago and completed a full reconnaissance before the smoke settled in. On the south (California) side, the leading southeastern edge of the fire continued to back downslope. -

Rickenella Swartzii (Fr.) Kuyper ROD Name Rickenella Setipes Family Tricholomataceae Morphological Habit Mushroom
S3 - 97 Rickenella swartzii (Fr.) Kuyper ROD name Rickenella setipes Family Tricholomataceae Morphological Habit mushroom Description: CAP 5-15 mm in diam., plano-convex, plano-umbilicate to deeply depressed, pellucid-striate to subsulcate, surface hygrophanous, moist, pruinose overall, dark violet- brown to dark sepia and margin vinaceous cinnamon, yellow-brown, becoming paler with moisture loss to deep brown-drab, violet gray or violet-brown on the disc, and margin pink- cinnamon, avellaneous or yellow-tan. GILLS deeply decurrent, in age becoming anastomosed, rugose or veined, white to pale cream, pruinose, edges concolorous, fimbriate. STEM 20-50 (-70) x 0.5-2 mm, central, cartilaginous, pruinose to pubescent overall or with base white-fibrillose, apex dark violet-brown, black-sepia or sordid violet-gray, base yellow-brown to pink-cinnamon. BASIDIA 15-22 x 4-5 µm, clavate, 4 spored. CHEILOCYSTIDIA scattered to abundant, 35-66 x 8-14 (-18) µm, ventricose-subcapitate to fusiform- subcapitate, hyaline. PLEUROCYSTIDIA scattered, similar to the cheilocystidia. PILEIPELLIS a cutis with numerous projecting pileocystidia. PILEOCYSTIDIA 50-90 x 8-18 µm, similar to the cheilocystidia. CAULOCYSTIDIA numerous, similar to cheilocystidia. CLAMP CONNECTIONS present. SPORES ellipsoid, (4-) 5-7 x 2-3 (-3.5) µm, smooth, hyaline, inamyloid, thin walled. Distinguishing Features: In the field it may look slightly similar to Omphalina pyxidata and Phytoconis ericetorum, but these species differ in lacking a violaceous cap disc and stem apex, and in lacking conspicuous cystidia on cap, gills, and stem. Distribution: Widespread across northern temperate forests. CALIFORNIA, Del Norte Co., Crescent City; OREGON, Lane Co., Siuslaw National Forest (SNF), Siltcoos River; Lincoln Co., SNF, Canal Creek; SNF, Five Rivers; WASHINGTON, King Co., University of Washington campus; Pierce Co., Mount Rainier National Park (MRNP), Longmire; MRNP, Tahoma Creek; Snohomish Co., Mount Baker-Snoqualmie National Forest, Barlow Pass; Meadowdale. -

Pacific Northwest Wilderness
pacific northwest wilderness for the greatest good * Throughout this guide we use the term Wilderness with a capital W to signify lands that have been designated by Congress as part of the National Wilderness Preservation System whether we name them specifically or not, as opposed to land that has a wild quality but is not designated or managed as Wilderness. Table of Contents Outfitter/Guides Are Wilderness Partners .................................................3 The Promise of Wilderness ............................................................................4 Wilderness in our Backyard: Pacific Northwest Wilderness ...................7 Wilderness Provides .......................................................................................8 The Wilderness Experience — What’s Different? ......................................9 Wilderness Character ...................................................................................11 Keeping it Wild — Wilderness Management ...........................................13 Fish and Wildlife in Wilderness .................................................................15 Fire and Wilderness ......................................................................................17 Invasive Species and Wilderness ................................................................18 Climate Change and Wilderness ................................................................19 Resources ........................................................................................................21 -

Cascades Frog Conservation Assessment
D E E P R A U R T LT MENT OF AGRICU United States Department of Agriculture Forest Service Pacific Southwest Research Station Cascades Frog General Technical Report PSW-GTR-244 Conservation Assessment March 2014 Karen Pope, Catherine Brown, Marc Hayes, Gregory Green, and Diane Macfarlane The U.S. Department of Agriculture (USDA) prohibits discrimination against its customers, employees, and applicants for employment on the bases of race, color, national origin, age, disability, sex, gender identity, religion, reprisal, and where applicable, political beliefs, marital status, familial or parental status, sexual orientation, or all or part of an individual’s income is derived from any public assistance program, or protected genetic information in employment or in any program or activity conducted or funded by the Department. (Not all prohibited bases will apply to all programs and/or employment activities.) If you wish to file an employment complaint, you must contact your agency’s EEO Counselor (PDF) within 45 days of the date of the alleged discriminatory act, event, or in the case of a personnel action. Additional information can be found online at http://www.ascr.usda.gov/complaint_filing_file.html. If you wish to file a Civil Rights program complaint of discrimination, complete the USDA Program Discrimination Complaint Form (PDF), found online at http://www.ascr.usda.gov/complaint_filing_cust. html, or at any USDA office, or call (866) 632-9992 to request the form. You may also write a letter containing all of the information requested in the form. Send your completed complaint form or letter to us by mail at U.S. -

Page 1480 TITLE 16—CONSERVATION § 1113 (Pub
§ 1113 TITLE 16—CONSERVATION Page 1480 (Pub. L. 88–363, § 13, July 7, 1964, 78 Stat. 301.) ment of expenses or salaries for the administra- tion of the National Wilderness Preservation § 1113. Authorization of appropriations System as a separate unit nor shall any appro- There are hereby authorized to be appro- priations be available for additional personnel priated to the Department of the Interior with- stated as being required solely for the purpose of out fiscal year limitation such sums as may be managing or administering areas solely because necessary for the purposes of this chapter and they are included within the National Wilder- the agreement with the Government of Canada ness Preservation System. signed January 22, 1964, article 11 of which pro- (c) ‘‘Wilderness’’ defined vides that the Governments of the United States A wilderness, in contrast with those areas and Canada shall share equally the costs of de- where man and his own works dominate the veloping and the annual cost of operating and landscape, is hereby recognized as an area where maintaining the Roosevelt Campobello Inter- the earth and its community of life are un- national Park. trammeled by man, where man himself is a visi- (Pub. L. 88–363, § 14, July 7, 1964, 78 Stat. 301.) tor who does not remain. An area of wilderness is further defined to mean in this chapter an CHAPTER 23—NATIONAL WILDERNESS area of undeveloped Federal land retaining its PRESERVATION SYSTEM primeval character and influence, without per- manent improvements or human habitation, Sec. which is protected and managed so as to pre- 1131. -

Mount Shasta Collection Pamphlet File Topics
College of the Siskiyous Library Mount Shasta Collection PAMPHLET FILE TOPICS Updated April 2019 MS PAMPHLET TOPICS A Abrams Lake (Calif.) Achomawi Indians Adams, Mount (Wash.) Aetherius Society Ager Ah-Di-Na Campground Algomah Camp Art - Crafts, Jewelry, etc. Art – Engraving / Lithographs, etc. Art - Graphic Art, Logos, Drawing Art - Murals Art - Visionary Art & Artists Art & Artists - General Art & Artists - A Art & Artists - B Art & Artists - C Art & Artists - D Art & Artists - E Art & Artists - F Art & Artists - G Art & Artists - H Art & Artists - I Art & Artists - J Art & Artists - K Art & Artists - L Art & Artists - M Art & Artists - N Art & Artists - O Art & Artists - P Art & Artists - Q Art & Artists - R Art & Artists - S Art & Artists - T Art & Artists - U Art & Artists - V Art & Artists - W-X Art & Artists - Y-Z Art & Artists - Unidentified Ascended Master Teaching Foundation Ash Creek (Calif) Association of Sananda and Sanat Kumara (Sister Thedra) Ashtar Command Ashtara Foundation Athapascan Language Audio-Visual (Movies) Audubon Endeavor (Mt. Shasta Area Audubon) Authors - Local Avalanche Gulch Avalanches MS PAMPHLET TOPICS B Baird Station Hatchery – Livingston Stone Baker, Mount (Wash.) Baxter, J.H. Bear Springs (Mt. Shasta) Berry Family Estate Bicycling Big Canyon Big Ditch Big Springs (Mayten) Big Springs - Mount Shasta City Park Big Springs - Shasta Valley Biological Survey of Mount Shasta Biology Biology - Life Zones Bioregion - Mount Shasta Black Bart Black Butte Bohemiam Club (San Francisco) Bolam Glacier (Mount Shasta)