Cascades Frog Conservation Assessment
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-
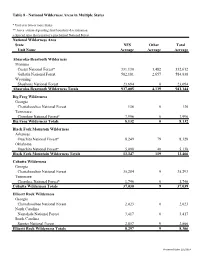
Table 8 — National Wilderness Areas in Multiple States
Table 8 - National Wilderness Areas in Multiple States * Unit is in two or more States ** Acres estimated pending final boundary determination + Special Area that is part of a proclaimed National Forest National Wilderness Area State NFS Other Total Unit Name Acreage Acreage Acreage Absaroka-Beartooth Wilderness Montana Custer National Forest* 331,130 1,482 332,612 Gallatin National Forest 582,181 2,657 584,838 Wyoming Shoshone National Forest 23,694 0 23,694 Absaroka-Beartooth Wilderness Totals 937,005 4,139 941,144 Big Frog Wilderness Georgia Chattahoochee National Forest 136 0 136 Tennessee Cherokee National Forest* 7,996 0 7,996 Big Frog Wilderness Totals 8,132 0 8,132 Black Fork Mountain Wilderness Arkansas Ouachita National Forest* 8,249 79 8,328 Oklahoma Ouachita National Forest* 5,098 40 5,138 Black Fork Mountain Wilderness Totals 13,347 119 13,466 Cohutta Wilderness Georgia Chattahoochee National Forest 35,284 9 35,293 Tennessee Cherokee National Forest* 1,746 0 1,746 Cohutta Wilderness Totals 37,030 9 37,039 Ellicott Rock Wilderness Georgia Chattahoochee National Forest 2,023 0 2,023 North Carolina Nantahala National Forest 3,417 0 3,417 South Carolina Sumter National Forest 2,857 9 2,866 Ellicott Rock Wilderness Totals 8,297 9 8,306 Processed Date: 2/5/2014 Table 8 - National Wilderness Areas in Multiple States * Unit is in two or more States ** Acres estimated pending final boundary determination + Special Area that is part of a proclaimed National Forest National Wilderness Area State NFS Other Total Unit Name Acreage Acreage -

State of Sierra Frogs
State of Sierra Frogs A report on the status of frogs & toads in the Sierra Nevada & California Cascade Mountains State of Sierra Frogs A report on the status of frogs & toads in the Sierra Nevada & California Cascade Mountains By Marion Gee, Sara Stansfield, & Joan Clayburgh July 2008 www.sierranevadaalliance.org State of Sierra Frogs 1 Acknowledgements The impetus for this report was the invaluable research on pesticides by Carlos Davidson, professor at San Francisco State University. Davidson, along with Amy Lind (US Forest Service), Curtis Milliron (California Department of Fish and Game), David Bradford (United States Environmental Protection Agency) and Kim Vincent (Graduate Student, San Francisco State University), generously donated their time and expertise to speak at two public workshops on the topics of Sierra frogs and toads as well as to provide comments for this document. Our thanks to the other reviewers of this manuscripts including Bob Stack (Jumping Frog Research Institute), Katie Buelterman, Dan Keenan, and Genevieve Jessop Marsh. This project was fortunate to receive contributions of photography and artwork from John Muir Laws, Elena DeLacy, Bob Stack, Ralph & Lisa Cutter and Vance Vredenburg. Photo credits are found with each caption. This work was made possible by generous grants from the Rose Foundation for Communities and the Environment and the State Water Resources Control Board. Funding for this project has been provided in part through an Agreement with the State Water Resources Control Board (SWRCB) pursuant to the Costa-Machado Water Act of 2000 (Proposition 13) and any amendments thereto for the implementation of California’s Non-point Source Pollution Control Program. -

Texas Co-Op Power • March 2017
LOCAL ELECTRIC COOPERATIVE EDITION MARCH 2017 Blizzard of ’57 Spring Vegetable Salads Palo Duro Pageant 35 35on Brake-worthy stops on highway through co-op country THE TRACTOR THAT STARTED IT ALL. IS CHANGING IT ALL. Metal Hood, Fenders New Deluxe Seat** New Swift-TTaach Loader** New Grille Guard New Dash Panel & Operator Platform & Tilt Steering Wheel & Swift-Connect Backhoe & Front Hitch & Display ALL-NEWW KUBOTTAA BX80 SERIES Low-Rate, Long-TTeerm Financing 6 YYeear Goingg On Noww! Limited Powertrain Warrantyy* kubota.com *Only terms and conditions of Kubota’s standard Limited Warranty applyy.. For warranty terms, see Kubota’s Limited Warranty at www.kubota.com or authorized Kubota Dealers. **Only avvaailable on certain models. Optional equipment may be shown. © Kubota TTrractor Corporation, 2017 Since 1944 March 2017 FAVORITES 5 Letters 6 Currents 18 Local Co-op News Get the latest information plus energy and safety tips from your cooperative. 29 Texas History Panhandle Blizzard of 1957 By Dawn Stephens 31 Recipes Spring Vegetable Salads 35 Focus on Texas Photo Contest: In Motion 36 Around Texas List of Local Events 38 Hit the Road Texas on a Grand Stage in Palo Duro By Sheryl Smith-Rodgers Marker near the northern end ONLINE of Interstate 35 in Texas, just TexasCoopPower.com south of the Red River Find these stories online if they don’t appear in your edition of the magazine. FEATURE Texas USA Odessa Meteor Crater 35 on 35 8 Stops with fascinating food, history and popular By E.R. Bills culture lure travelers from the interstate as it weaves Observations through Texas from Mexico to Oklahoma Another Roadside Attraction Story and photos by Julia Robinson By Ryann Ford ROAD TRIP! See video and photos at TexasCoopPower.com NEXT MONTH Drones: An Overview Texas inno- vators, including electric co-ops, hone drones as tools of today. -

Copyrighted Material
Index Abulfeda crater chain (Moon), 97 Aphrodite Terra (Venus), 142, 143, 144, 145, 146 Acheron Fossae (Mars), 165 Apohele asteroids, 353–354 Achilles asteroids, 351 Apollinaris Patera (Mars), 168 achondrite meteorites, 360 Apollo asteroids, 346, 353, 354, 361, 371 Acidalia Planitia (Mars), 164 Apollo program, 86, 96, 97, 101, 102, 108–109, 110, 361 Adams, John Couch, 298 Apollo 8, 96 Adonis, 371 Apollo 11, 94, 110 Adrastea, 238, 241 Apollo 12, 96, 110 Aegaeon, 263 Apollo 14, 93, 110 Africa, 63, 73, 143 Apollo 15, 100, 103, 104, 110 Akatsuki spacecraft (see Venus Climate Orbiter) Apollo 16, 59, 96, 102, 103, 110 Akna Montes (Venus), 142 Apollo 17, 95, 99, 100, 102, 103, 110 Alabama, 62 Apollodorus crater (Mercury), 127 Alba Patera (Mars), 167 Apollo Lunar Surface Experiments Package (ALSEP), 110 Aldrin, Edwin (Buzz), 94 Apophis, 354, 355 Alexandria, 69 Appalachian mountains (Earth), 74, 270 Alfvén, Hannes, 35 Aqua, 56 Alfvén waves, 35–36, 43, 49 Arabia Terra (Mars), 177, 191, 200 Algeria, 358 arachnoids (see Venus) ALH 84001, 201, 204–205 Archimedes crater (Moon), 93, 106 Allan Hills, 109, 201 Arctic, 62, 67, 84, 186, 229 Allende meteorite, 359, 360 Arden Corona (Miranda), 291 Allen Telescope Array, 409 Arecibo Observatory, 114, 144, 341, 379, 380, 408, 409 Alpha Regio (Venus), 144, 148, 149 Ares Vallis (Mars), 179, 180, 199 Alphonsus crater (Moon), 99, 102 Argentina, 408 Alps (Moon), 93 Argyre Basin (Mars), 161, 162, 163, 166, 186 Amalthea, 236–237, 238, 239, 241 Ariadaeus Rille (Moon), 100, 102 Amazonis Planitia (Mars), 161 COPYRIGHTED -

Castle Crags State Park Brochure
Our Mission The mission of California State Parks is Castle Crags to provide for the health, inspiration and education of the people of California by helping he lofty spires and to preserve the state’s extraordinary biological T State Park diversity, protecting its most valued natural and granite dome of Castle Crags cultural resources, and creating opportunities for high-quality outdoor recreation. rise to more than 6,500 feet. The grandeur of the crags has been revered as California State Parks supports equal access. an extraordinary place Prior to arrival, visitors with disabilities who need assistance should contact the park for millennia. at (530) 235-2684. This publication can be made available in alternate formats. Contact [email protected] or call (916) 654-2249. CALIFORNIA STATE PARKS P.O. Box 942896 Sacramento, CA 94296-0001 For information call: (800) 777-0369 (916) 653-6995, outside the U.S. 711, TTY relay service www.parks.ca.gov Discover the many states of California.™ Castle Crags State Park 20022 Castle Creek Road Castella, CA 96017 (530) 235-2684 © 2014 California State Parks M ajestic Castle Crags have inspired The Okwanuchu Shasta territory covered A malaria epidemic brought by European fur enduring myths and legends since about 700 square miles of forested mountains trappers wiped out much of the Okwanuchu prehistoric times. More than 170 million from the headwaters of the Sacramento River Shasta populace by 1833. years old, these granite formations in to the McCloud River and from Mount Shasta With the 1848 gold discoveries at the the Castle Crags Wilderness border the to Pollard Flat. -

Land Areas of the National Forest System, As of September 30, 2019
United States Department of Agriculture Land Areas of the National Forest System As of September 30, 2019 Forest Service WO Lands FS-383 November 2019 Metric Equivalents When you know: Multiply by: To fnd: Inches (in) 2.54 Centimeters Feet (ft) 0.305 Meters Miles (mi) 1.609 Kilometers Acres (ac) 0.405 Hectares Square feet (ft2) 0.0929 Square meters Yards (yd) 0.914 Meters Square miles (mi2) 2.59 Square kilometers Pounds (lb) 0.454 Kilograms United States Department of Agriculture Forest Service Land Areas of the WO, Lands National Forest FS-383 System November 2019 As of September 30, 2019 Published by: USDA Forest Service 1400 Independence Ave., SW Washington, DC 20250-0003 Website: https://www.fs.fed.us/land/staff/lar-index.shtml Cover Photo: Mt. Hood, Mt. Hood National Forest, Oregon Courtesy of: Susan Ruzicka USDA Forest Service WO Lands and Realty Management Statistics are current as of: 10/17/2019 The National Forest System (NFS) is comprised of: 154 National Forests 58 Purchase Units 20 National Grasslands 7 Land Utilization Projects 17 Research and Experimental Areas 28 Other Areas NFS lands are found in 43 States as well as Puerto Rico and the Virgin Islands. TOTAL NFS ACRES = 192,994,068 NFS lands are organized into: 9 Forest Service Regions 112 Administrative Forest or Forest-level units 503 Ranger District or District-level units The Forest Service administers 149 Wild and Scenic Rivers in 23 States and 456 National Wilderness Areas in 39 States. The Forest Service also administers several other types of nationally designated -

No. 40. the System of Lunar Craters, Quadrant Ii Alice P
NO. 40. THE SYSTEM OF LUNAR CRATERS, QUADRANT II by D. W. G. ARTHUR, ALICE P. AGNIERAY, RUTH A. HORVATH ,tl l C.A. WOOD AND C. R. CHAPMAN \_9 (_ /_) March 14, 1964 ABSTRACT The designation, diameter, position, central-peak information, and state of completeness arc listed for each discernible crater in the second lunar quadrant with a diameter exceeding 3.5 km. The catalog contains more than 2,000 items and is illustrated by a map in 11 sections. his Communication is the second part of The However, since we also have suppressed many Greek System of Lunar Craters, which is a catalog in letters used by these authorities, there was need for four parts of all craters recognizable with reasonable some care in the incorporation of new letters to certainty on photographs and having diameters avoid confusion. Accordingly, the Greek letters greater than 3.5 kilometers. Thus it is a continua- added by us are always different from those that tion of Comm. LPL No. 30 of September 1963. The have been suppressed. Observers who wish may use format is the same except for some minor changes the omitted symbols of Blagg and Miiller without to improve clarity and legibility. The information in fear of ambiguity. the text of Comm. LPL No. 30 therefore applies to The photographic coverage of the second quad- this Communication also. rant is by no means uniform in quality, and certain Some of the minor changes mentioned above phases are not well represented. Thus for small cra- have been introduced because of the particular ters in certain longitudes there are no good determi- nature of the second lunar quadrant, most of which nations of the diameters, and our values are little is covered by the dark areas Mare Imbrium and better than rough estimates. -

Wood Frog (Rana Sylvatica): a Technical Conservation Assessment
Wood Frog (Rana sylvatica): A Technical Conservation Assessment Prepared for the USDA Forest Service, Rocky Mountain Region, Species Conservation Project March 24, 2005 Erin Muths1, Suzanne Rittmann1, Jason Irwin2, Doug Keinath3, Rick Scherer4 1 U.S. Geological Survey, Fort Collins Science Center, 2150 Centre Ave. Bldg C, Fort Collins, CO 80526 2 Department of Biology, Bucknell University, Lewisburg, PA 17837 3 Wyoming Natural Diversity Database, University of Wyoming, P.O. Box 3381, Laramie, WY 82072 4 Colorado State University, GDPE, Fort Collins, CO 80524 Peer Review Administered by Society for Conservation Biology Muths, E., S. Rittman, J. Irwin, D. Keinath, and R. Scherer. (2005, March 24). Wood Frog (Rana sylvatica): a technical conservation assessment. [Online]. USDA Forest Service, Rocky Mountain Region. Available: http://www.fs.fed.us/r2/projects/scp/assessments/woodfrog.pdf [date of access]. ACKNOWLEDGMENTS The authors would like to acknowledge the help of the many people who contributed time and answered questions during our review of the literature. AUTHORS’ BIOGRAPHIES Dr. Erin Muths is a Zoologist with the U.S. Geological Survey – Fort Collins Science Center. She has been studying amphibians in Colorado and the Rocky Mountain Region for the last 10 years. Her research focuses on demographics of boreal toads, wood frogs and chorus frogs and methods research. She is a principle investigator for the USDOI Amphibian Research and Monitoring Initiative and is an Associate Editor for the Northwestern Naturalist. Dr. Muths earned a B.S. in Wildlife Ecology from the University of Wisconsin, Madison (1986); a M.S. in Biology (Systematics and Ecology) from Kansas State University (1990) and a Ph.D. -

Rana Temporaria)
European Community Directive on the Conservation of Natural Habitats and of Wild Fauna and Flora (92/43/EEC) Fourth Report by the United Kingdom under Article 17 on the implementation of the Directive from January 2013 to December 2018 Supporting documentation for the conservation status assessment for the species: S1213 ‐ Common frog (Rana temporaria) ENGLAND IMPORTANT NOTE ‐ PLEASE READ • The information in this document is a country‐level contribution to the UK Reporton the conservation status of this species, submitted to the European Commission aspart of the 2019 UK Reporting under Article 17 of the EU Habitats Directive. • The 2019 Article 17 UK Approach document provides details on how this supporting information was used to produce the UK Report. • The UK Report on the conservation status of this species is provided in a separate doc‐ ument. • The reporting fields and options used are aligned to those set out in the European Com‐ mission guidance. • Explanatory notes (where provided) by the country are included at the end. These pro‐ vide an audit trail of relevant supporting information. • Some of the reporting fields have been left blank because either: (i) there was insuffi‐ cient information to complete the field; (ii) completion of the field was not obligatory; (iii) the field was not relevant to this species (section 12 Natura 2000 coverage forAnnex II species) and/or (iv) the field was only relevant at UK‐level (sections 9 Future prospects and 10 Conclusions). • For technical reasons, the country‐level future trends for Range, Population and Habitat for the species are only available in a separate spreadsheet that contains all the country‐ level supporting information. -

DNR Letterhead
ATU F N RA O L T R N E E S M O T U STATE OF MICHIGAN R R C A P DNR E E S D MI N DEPARTMENT OF NATURAL RESOURCES CHIG A JENNIFER M. GRANHOLM LANSING REBECCA A. HUMPHRIES GOVERNOR DIRECTOR Michigan Frog and Toad Survey 2009 Data Summary There were 759 unique sites surveyed in Zone 1, 218 in Zone 2, 20 in Zone 3, and 100 in Zone 4, for a total of 1097 sites statewide. This is a slight decrease from the number of sites statewide surveyed last year. Zone 3 (the eastern half of the Upper Peninsula) is significantly declining in routes. Recruiting in that area has become necessary. A few of the species (i.e. Fowler’s toad, Blanchard’s cricket frog, and mink frog) have ranges that include only a portion of the state. As was done in previous years, only data from those sites within the native range of those species were used in analyses. A calling index of abundance of 0, 1, 2, or 3 (less abundant to more abundant) is assigned for each species at each site. Calling indices were averaged for a particular species for each zone (Tables 1-4). This will vary widely and cannot be considered a good estimate of abundance. Calling varies greatly with weather conditions. Calling indices will also vary between observers. Results from the evaluation of methods and data quality showed that volunteers were very reliable in their abilities to identify species by their calls, but there was variability in abundance estimation (Genet and Sargent 2003). -

Factors Related to the Distribution and Prevalence
Biological Conservation 144 (2011) 2913–2921 Contents lists available at SciVerse ScienceDirect Biological Conservation journal homepage: www.elsevier.com/locate/biocon Factors related to the distribution and prevalence of the fungal pathogen Batrachochytrium dendrobatidis in Rana cascadae and other amphibians in the Klamath Mountains ⇑ Jonah Piovia-Scott a,b, , Karen L. Pope c, Sharon P. Lawler a,d, Esther M. Cole e, Janet E. Foley b a Center for Population Biology, University of California – Davis, One Shields Avenue, Davis, CA 95616, USA b Department of Veterinary Medicine and Epidemiology, University of California – Davis, One Shields Avenue, Davis, CA 95616, USA c United States Forest Service, Pacific Southwest Research Station, 1700 Bayview Drive, Arcata, CA 95521, USA d Department of Entomology, University of California – Davis, One Shields Avenue, Davis, CA 95616, USA e Department of Environmental Science and Policy, University of California – Davis, One Shields Avenue, Davis, CA 95616, USA article info abstract Article history: The fungal pathogen Batrachochytrium dendrobatidis (Bd), which causes the disease chytridiomycosis, has Received 5 May 2011 been associated with declines and extinctions of montane amphibians worldwide. To gain insight into Received in revised form 29 July 2011 factors affecting its distribution and prevalence we focus on the amphibian community of the Klamath Accepted 22 August 2011 Mountains in northwest California. The Cascades frog (Rana cascadae), one of the most common amphib- Available online 5 October 2011 ians in these mountains, experienced increased mortality as a result of Bd exposure in laboratory trials and has experienced recent, dramatic declines in other parts of California. We surveyed 112 sites in Keywords: the Klamaths, all of which supported R. -

Table 8 - National Wilderness Areas in Multiple States
Table 8 - National Wilderness Areas in Multiple States * Unit is in two or more States ** Acres estimated pending final boundary determination + Special Area that is part of a proclaimed National Forest National Wilderness Area State NFS Other Total Unit Name Acreage Acreage Acreage Absaroka-Beartooth Wilderness Montana Custer National Forest* 331,130 1,482 332,612 Gallatin National Forest 582,181 2,657 584,838 Wyoming Shoshone National Forest 23,694 0 23,694 Absaroka-Beartooth Wilderness Total 937,005 4,139 941,144 Big Frog Wilderness Georgia Chattahoochee National Forest 136 0 136 Tennessee Cherokee National Forest* 7,996 0 7,996 Big Frog Wilderness Total 8,132 0 8,132 Black Fork Mountain Wilderness Arkansas Ouachita National Forest* 8,249 79 8,328 Oklahoma Ouachita National Forest* 5,098 40 5,138 Black Fork Mountain Wilderness Total 13,347 119 13,466 Cohutta Wilderness Georgia Chattahoochee National Forest 35,284 9 35,293 Tennessee Cherokee National Forest* 1,746 0 1,746 Cohutta Wilderness Total 37,030 9 37,039 Ellicott Rock Wilderness Georgia Chattahoochee National Forest 2,023 0 2,023 North Carolina Nantahala National Forest 3,417 0 3,417 South Carolina Sumter National Forest 2,857 9 2,866 Ellicott Rock Wilderness Total 8,297 9 8,306 Refresh Date: 10/18/2014 Table 8 - National Wilderness Areas in Multiple States * Unit is in two or more States ** Acres estimated pending final boundary determination + Special Area that is part of a proclaimed National Forest National Wilderness Area State NFS Other Total Unit Name Acreage Acreage Acreage