Chemexplained.Com Master Lab Equipment and Supplies List (Through Lab #18)
Total Page:16
File Type:pdf, Size:1020Kb

Load more
Recommended publications
-

LAB: One Tube Reaction Part 1
AP Chemistry LAB: One Tube Reaction Part 1 Objective: To monitor and document the chemical changes occurring in a single test tube containing a predetermined mixture of chemicals. Materials: test tube, iron nail, sodium chloride, copper (II) sulfate, distilled water, glass stirring rod, tissue paper, parafilm wax Procedures: 1. Label a test tube to identify your group 2. Fill approx. 1/3 of the test tube with copper (II) sulfate crystals. Gently tap the tube to allow the crystals to settle. 3. Using a glass stirring rod, carefully cover the crystals with a layer of Kimwipe tissue paper. 4. Slowly, and with as little disturbance as possible, add enough distilled water to just cover the paper and blue crystals. 5. Repeat the process for the sodium chloride, filling approx. 1/3 of the test tube. Gently tap the tube to allow the crystals to settle. 6. Push more tissue paper into the test tube on top of the white crystals. 7. Add enough water to cover the tissue paper and white crystals. 8. Obtain an iron nail and expose the surface by rubbing with sand paper. 9. Carefully slide the nail into the test tube. 10. Continue adding water until the nail is completely covered. 11. Cover the test tube with parafilm wax and record your Day 1 observations. 12. Return the test tube to the test tube rack. 13. Continue to monitor and record your observations for the next several days, as indicated in the data table. Data: 1. Complete the NFPA label for the two chemicals used in this lab. -

Equipment Detailsr07
Lab Equipment Details Lab Equipment Glass Flasks 150ml 250ml 500ml Lab Equipment Glass Beakers 150ml 250ml 500ml Lab Equipment Glassware But once removed, only the cap stays highlighted. Droppers critical to Lab An activated Dropper coursework can be found highlights the entire bottle. already in the workspace. Dropper Dropper Dropper Activated In Use Lab Equipment Gastight Syringe Small A pre-filled gastight syringe can be used with a NMR tube to safely fill through the top in preparation for use with the NMR spectrometer. Gastight Syringe NMR Tube with Holder with Holder Lab Equipment NMR Tube Spinner An NMR Tube filled with gas for use with the NMR Spectrometer Simply use the NMR Tube with needs to be inserted into the the Tube Spinner. Spinner before it can be used. NMR Tube NMR Tube Spinner NMR Tube Spinner with Holder with NMR Tube inserted Once the Tube slotted into the holder is inserted into the top part of the spectrometer, users can type in a number for Lab Equipment the frequency they’d like to scan. NMR Spectrometer XL The NMR Tube holder can then be slotted into the highlighted tube on the NMR Spectrometer. NMR Tube filled with appropriate substance is slotted (used with) in the holder. Lift will perpare the Spectrometer for the tube holder insertion. Scan No. Allows the user to change the frequency at which the tube is scanned The NMR Tube holder can then be slotted into the top tube on Lab Equipment the NMR Spectrometer. NMR Spectrometer XL NMR Tube filled with appropriate substance is slotted (used with) in the holder. -

General Chemistry Laboratory I Manual
GENERAL CHEMISTRY LABORATORY I MANUAL Fall Semester Contents Laboratory Equipments .............................................................................................................................. i Experiment 1 Measurements and Density .............................................................................................. 10 Experiment 2 The Stoichiometry of a Reaction ..................................................................................... 31 Experiment 3 Titration of Acids and Bases ............................................................................................ 10 Experiment 4 Oxidation – Reduction Titration ..................................................................................... 49 Experiment 5 Quantitative Analysis Based on Gas Properties ............................................................ 57 Experiment 6 Thermochemistry: The Heat of Reaction ....................................................................... 67 Experiment 7 Group I: The Soluble Group ........................................................................................... 79 Experiment 8 Gravimetric Analysis ........................................................................................................ 84 Scores of the General Chemistry Laboratory I Experiments ............................................................... 93 LABORATORY EQUIPMENTS BEAKER (BEHER) Beakers are containers which can be used for carrying out reactions, heating solutions, and for water baths. They are for -

Laboratory Equipment Reference Sheet
Laboratory Equipment Stirring Rod: Reference Sheet: Iron Ring: Description: Glass rod. Uses: To stir combinations; To use in pouring liquids. Evaporating Dish: Description: Iron ring with a screw fastener; Several Sizes Uses: To fasten to the ring stand as a support for an apparatus Description: Porcelain dish. Buret Clamp/Test Tube Clamp: Uses: As a container for small amounts of liquids being evaporated. Glass Plate: Description: Metal clamp with a screw fastener, swivel and lock nut, adjusting screw, and a curved clamp. Uses: To hold an apparatus; May be fastened to a ring stand. Mortar and Pestle: Description: Thick glass. Uses: Many uses; Should not be heated Description: Heavy porcelain dish with a grinder. Watch Glass: Uses: To grind chemicals to a powder. Spatula: Description: Curved glass. Uses: May be used as a beaker cover; May be used in evaporating very small amounts of Description: Made of metal or porcelain. liquid. Uses: To transfer solid chemicals in weighing. Funnel: Triangular File: Description: Metal file with three cutting edges. Uses: To scratch glass or file. Rubber Connector: Description: Glass or plastic. Uses: To hold filter paper; May be used in pouring Description: Short length of tubing. Medicine Dropper: Uses: To connect parts of an apparatus. Pinch Clamp: Description: Glass tip with a rubber bulb. Uses: To transfer small amounts of liquid. Forceps: Description: Metal clamp with finger grips. Uses: To clamp a rubber connector. Test Tube Rack: Description: Metal Uses: To pick up or hold small objects. Beaker: Description: Rack; May be wood, metal, or plastic. Uses: To hold test tubes in an upright position. -

2019 Beverage Industry Supplies Catalog Table of Contents
2019 Beverage Industry Supplies Catalog Table of Contents Barrels, Racks & Wood Products……………………………………………………………...4 Chemicals Cleaners and Sanitizers…………………………………………………………..10 Processing Chemicals……………………………………………………………..13 Clamps, Fittings & Valves……………………………………………………………………….14 Fermentation Bins…………………………………………………………………………………18 Filtration Equipment and Supplies……...…………………………………………………..19 Fining Agents………………………………………………………………………………………..22 Hoses…………………………………………………………………………………………………..23 Laboratory Assemblies & Kits…………………………………………………………………..25 Chemicals……………………………………………………………………………..28 Supplies………………………………………………………………………………..29 Testers………………………………………………………………………………… 37 Malo-Lactic Bacteria & Nutrients…………………………………………………………….43 Munton’s Malts……………………………………………………………………………………..44 Packaging Products Bottles, Bottle Wax, Capsules………………………………………………….45 Natural Corks………………………………………………………………………..46 Synthetic Corks……………………………………………………………………..47 Packaging Equipment…………………………………………………………………………….48 Pumps………………………………………………………………………………………………….50 Sulfiting Agents…………………………………………………………………………………….51 Supplies……………………………………………………………………………………………….52 Tanks…………………………………………………………………………………………………..57 Tank Accessories…………………………………………………………………………………..58 Tannins………………………………………………………………………………………………..59 Yeast, Nutrient & Enzymes……………………………………………………………………..61 Barrels, Racks & Wood Products Barrels Description Size Price LeRoi, New French Oak 59 gl Call for Pricing Charlois, New American Oak 59 gl Call for Pricing Charlois, New Hungarian Oak 59 gl Call for Pricing Used -

Standard Methods for the Examination of Water and Wastewater
Standard Methods for the Examination of Water and Wastewater Part 1000 INTRODUCTION 1010 INTRODUCTION 1010 A. Scope and Application of Methods The procedures described in these standards are intended for the examination of waters of a wide range of quality, including water suitable for domestic or industrial supplies, surface water, ground water, cooling or circulating water, boiler water, boiler feed water, treated and untreated municipal or industrial wastewater, and saline water. The unity of the fields of water supply, receiving water quality, and wastewater treatment and disposal is recognized by presenting methods of analysis for each constituent in a single section for all types of waters. An effort has been made to present methods that apply generally. Where alternative methods are necessary for samples of different composition, the basis for selecting the most appropriate method is presented as clearly as possible. However, samples with extreme concentrations or otherwise unusual compositions or characteristics may present difficulties that preclude the direct use of these methods. Hence, some modification of a procedure may be necessary in specific instances. Whenever a procedure is modified, the analyst should state plainly the nature of modification in the report of results. Certain procedures are intended for use with sludges and sediments. Here again, the effort has been to present methods of the widest possible application, but when chemical sludges or slurries or other samples of highly unusual composition are encountered, the methods of this manual may require modification or may be inappropriate. Most of the methods included here have been endorsed by regulatory agencies. Procedural modification without formal approval may be unacceptable to a regulatory body. -

Documm Mgson!
Documm mgson! ED 174 412 SE 027 981 TITLE Biomedical Science, Unit I: Respiration inHealth and Medicine. Respiratory Anatomy, Physiology and Pathology; The Behavior of Gases;Introductory Chemistry; and Air Pollution. Laboratory Manual. Revised Version, 1975. INSTITUTION Biomedical Interdisciplinary Curriculum Project, Berkeley, Calif. SPONS AGENCY National Science Foundation, Washington, D.C. PUB DATE 75 NOTE 181p.; For related documents, see SE 027978-999 and SE 028 510-516; Not available in hard copydue to copyright restrictions EDRS PRICE MF01 Plus Postage. PC Not Available from EDRS. DESCRIpTCRS Chemistry; Environment; Health; *HealthEducation; Higher Education; *Laboratory ExFeriments; *Laboratory Techniques; *Laboratory Training;Medical Education; Pathology; Physics; *Physiology;*Science Education; Secondary Education IDENTIFIERS *Respiration ABSTItACT Designed to accompany the student text on respiration, this manual presents instructions on the use of labcra tcry equipment and presents various experimentsdealing with the concepts presented in the text. Thirty=nine laboratoryactivities ' are de scribed. Laboratory activities are dividedinto several parts, each Part covering a specific experiment dealing with the concept covered by the activity. Each experimentincludes a description of materials, procedures, and discurision questions. (RE) ********************************************************************** Reproductions supplied by EDRS are the bestthat can be made from the original document. 4********************M414141#***********4141*****4141***********41#********4 -
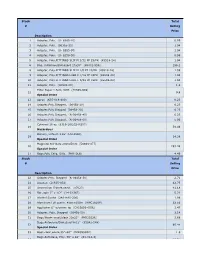
Stock Total # Selling Price 1 Adapter, Poly. (U- 6365-10)
Stock Total # Selling Price Description 1 Adapter, Poly. (U- 6365-10) 0.99 2 Adapter, Poly. (06365-22) 1.04 3 Adapter, Poly. (U- 6365-30) 1.04 4 Adapter, Poly. (U- 6359-50) 0.99 5 Adapter, Poly,FITTINGS SLIP M 3/32 PP 25/PK (45518-24) 1.04 6 Bag, Autoclave/Biohazard 24x30'' (95042-556) 156.1 8 Adapter, Poly.FITTINGS SLIP M 1/8 PP 25/PK (45518-26) 1.59 9 Adapter, Poly.FITTINGS LUER F 1/16 PP 25PK (45508-00) 1.04 10 Adapter, Poly.FITTINGS LUER F 3/32 PP 25PK (45508-02) 1.04 11 Adapter, Poly. (30800-08) 1.3 Filter Paper 1.5cm, VWR (28309-989) 12 9.9 Special Order 13 Apron (635-018-400) 0.23 14 Adapter,Poly.Stepped, (06458-10) 6.23 15 Adapter,Poly.Stepped (06458-20) 6.23 16 Adapter,Poly.Stepped, (N-06458-40) 6.23 17 Adapter,Poly.Stepped, (N-06458-60) 5.06 Cytoseal 16 oz (8310-16/23244257) 18 58.49 Hazardous Battery, Lithium 3.6V (LS14500) 19 14.38 Special Order Magnetic Stir Bars 2mmx5mm (58948-377) 20 293.48 Special Order 21 Bags,Poly Cello,10 lb. (PKR10Lb) 4.49 Stock Total # Selling Price Description 22 Adapter,Poly. Stepped (N-06458-30) 2.71 24 Alconox (21835-032) 42.75 25 Ammonium Sulfate,certif. (A7023) 41.18 26 Bar, spin 2'' x 1/2'' (14-51367) 5.22 27 Alcohol Swabs (248-HAS-200) 1.96 28 Aluminum Foil comm. 45cmx100m (WPC1810P) 32.56 29 Applicator 6'' w/cotton tip (CA10806-000L) 1.47 30 Adapter, Poly. -

Laboratory Equipment Used in Filtration
KNOW YOUR LAB EQUIPMENTS Test tube A test tube, also known as a sample tube, is a common piece of laboratory glassware consisting of a finger-like length of glass or clear plastic tubing, open at the top and closed at the bottom. Beakers Beakers are used as containers. They are available in a variety of sizes. Although they often possess volume markings, these are only rough estimates of the liquid volume. The markings are not necessarily accurate. Erlenmeyer flask Erlenmeyer flasks are often used as reaction vessels, particularly in titrations. As with beakers, the volume markings should not be considered accurate. Volumetric flask Volumetric flasks are used to measure and store solutions with a high degree of accuracy. These flasks generally possess a marking near the top that indicates the level at which the volume of the liquid is equal to the volume written on the outside of the flask. These devices are often used when solutions containing dissolved solids of known concentration are needed. Graduated cylinder Graduated cylinders are used to transfer liquids with a moderate degree of accuracy. Pipette Pipettes are used for transferring liquids with a fixed volume and quantity of liquid must be known to a high degree of accuracy. Graduated pipette These Pipettes are calibrated in the factory to release the desired quantity of liquid. Disposable pipette Disposable transfer. These Pipettes are made of plastic and are useful for transferring liquids dropwise. Burette Burettes are devices used typically in analytical, quantitative chemistry applications for measuring liquid solution. Differing from a pipette since the sample quantity delivered is changeable, graduated Burettes are used heavily in titration experiments. -
Complete Mill-Rose Product Catalog
BrushesBrushes ForFor IndustryIndustry MILL-ROSE offers industry a complete range of industrial brushes and is the largest manufacturer of twisted wire brushes in the country. Our 64,000 sq.ft. plant features the latest manufacturing techniques and quality control programs to assure unvarying high quality through- out our vast product line. This catalog contains hundreds of standard industrial brushes in stock and ready for shipment. These brushes are only a sample of the thousands of brushes Mill-Rose manufactures. Contact Mill-Rose if your brush requirements are special and not covered by this catalog. Our Specialty... CUSTOM-DESIGNED BRUSHES If you require a special or unique brush, we’ll design it for you and make it from any material the job calls for. MILL-ROSE has designed more than 100,000 special brushes with unique configurations for unique applications. Many of these brushes are brushes that don’t brush, or “un- brushes”. Un-brushes are used in all types of manufacturing, the medical field, vibration control, food processing, material handling, and hundreds of diverse applications where off-the-shelf brushes simply don’t work. Put our 75+ years of brush design/manufacturing experience to work for you. Tell us what you want to accomplish, or send us a sketch showing what you need. Our full-service engineering department will take it from there. It’s that easy. We’re great problem solvers. Side-Action Honing Tool Medical Instruments Mixing/Screening Device 2 HOW TO ORDER This catalog meets standards set by NIDA-SIDA and readily adapts itself to data processing systems. -

Chemistry 50 and 51 Laboratory Manual General Information
Chemistry 50 and 51 Laboratory Manual General Information Mt. San Antonio College Chemistry Department 2019 - 2020 TABLE OF CONTENTS PREFACE………………………………………………………………………..………..… 1 GENERAL INFORMATION Safety………………..………………………..…………………………………INFORMATION….……… 3 Equipment……………………………………..……………………………….………….. 9 Techniques………………………………………………………………………………… 13 Heating……………………………………...……………………………….…..….… 13 Cleaning and Labeling Glassware……….……………………….........……........…... 14 Reading Analog Scales…………………………………………………..……….…... 14 Volumetric Flasks………………………………………………………..……….…... 15 Graduated Cylinders.………………………………………………………………..… 15 Volumetric Pipets……………………………………..…………………………..…... 16 Graduated Pipets……………….……………………..………………………….…… 16 Burets………………………….………………..………………..………..….………. 18 Analytical Balances…………………………………………………….……….…….. 19 Solution Preparation…………………………………………..……………….….…... 20 Percent Concentration....……………………………………………….……….…….. 20 Molarity……………………………………………………………………………….. 21 Dilution……………………………………………………………...………………… 22 Titration ………………………………………………………………....…………..... 23 Vacuum Filtration……………………….…………………………………..…….…… 24 Spectrophotometry and Beer’s Law…………………………………..…………..…... 25 Measurement of pH………………………………………………….….….……..…... 27 Pasco Spectrometer……………………………………..…………………...….…… 28 Vernier Go Direct Sensors ………………………..…………………...….………….. 31 Notebook…………………………………………………………….….………..……...... 35 Precision and Accuracy……………………………………………………….………....... 37 Spreadsheet and Graphing with Excel…..…………………..…………..……………....... 46 EXPERIMENTS PREFACE The laboratory -

Laboratory Equipment AP
\ \\ , f ?7-\ Watch glass 1 Crucible and cover Evaporating dish Pneumatlo trough Beaker Safety goggles Florence Wide-mouth0 Plastic wash Dropper Funnel flask collecting bottle pipet Edenmeyer Rubber stoppers bottle flask € ....... ">. ÿ ,, Glass rod with niohrome wire Scoopula (for flame re,sting) CruoiNe tongs Rubber ubing '1 ,v .... Test-tube brush square Wire gau ÿ "\ file Burner " Tripod Florence flask: glass; common sizes are 125 mL, 250 mL, 500 .d Beaker: glass or plastic; common sizes are 50 mL, mL; maybe heated; used in making and for storing solutions. 100 mL, 250 mL, 400 mL; glass beakers maybe heated. oÿ Buret: glass; common sizes are 25 mL and 50 mL; used to Forceps: metal; used to hold or pick up small objects. Funnel: glass or plastic; common size holds 12.5-cm diameter measure volumes of solutions in titrafions. Ceramic square: used under hot apparatus or glassware. filter paper. Gas burner: constructed of metal; connected to a gas supply Clamps" the following types of clamps may be fastened to with rubber tubing; used to heat chemicals (dry or in solution) support apparatus: buret/test-tube clamp, clamp holder, double buret clamp, ring clamp, 3-pronged jaw clamp. in beakers, test tubes, and crucibles. Gas collecting tube: glass; marked in mL intervals; used to 3: Clay triangle: wire frame with porcelain supports; used to o} support a crucible. measure gas volumes. Glass rod with nichrome wire: used in flame tests. Condenser: glass; used in distillation procedures. Q. Crucible and cover: porcelain; used to heat small amounts of Graduated cylinder: glass or plastic; common sizes are 10 mL, 50 mL, 100 mL; used to measure approximate volumes; must solid substances at high temperatures.