Electrospray Tandem Mass Spectrometric Investigations of Morphinans
Total Page:16
File Type:pdf, Size:1020Kb

Load more
Recommended publications
-

Alternative Formats If You Require This Document in an Alternative Format, Please Contact: [email protected]
University of Bath PHD The extraction and chemistry of the metabolites of Mimosa tenuiflora and Papaver somniferum Ninan, Aleyamma Award date: 1990 Awarding institution: University of Bath Link to publication Alternative formats If you require this document in an alternative format, please contact: [email protected] General rights Copyright and moral rights for the publications made accessible in the public portal are retained by the authors and/or other copyright owners and it is a condition of accessing publications that users recognise and abide by the legal requirements associated with these rights. • Users may download and print one copy of any publication from the public portal for the purpose of private study or research. • You may not further distribute the material or use it for any profit-making activity or commercial gain • You may freely distribute the URL identifying the publication in the public portal ? Take down policy If you believe that this document breaches copyright please contact us providing details, and we will remove access to the work immediately and investigate your claim. Download date: 23. Sep. 2021 THE EXTRACTION AND CHEMISTRY OF THE METABOLITES OF MIMOSA TENUIFLORA AND PAP AVER SOMNIFERUM. submitted by ALEYAMMA NINAN for the degree of Doctor of Philosophy of the University of Bath 1990 Attention is drawn to the fact that the copyright of this thesis rests with its author. This copy of the thesis has been supplied on condition that anyone who consults it is understood to recognise that its copyright rests with its author and that no quotation from the thesis and no information derived from it may be published without prior consent of the author. -

The Metamorphosis of Hydromorphone Gary M
Pharmacy PersPective The metamorphosis of hydromorphone Gary M. Reisfield, MD George R. Wilson, MD introduction “That’s it. The Mayo Clinic at Rochester devel - oped it, the word and the drug, for it means a Hydromorphone hydrochloride, one of the oldest of drug, a pain relieving drug, five times as potent the extant opioid analgesics, has been in clinical use for as morphine, as harmless as water and with no more than 70 years. Its use by the oral route in chronic habit forming qualities. pain and hospice/palliative medicine settings has been limited, however, largely owing to its relatively short “The medical journals say it is particularly useful duration of action. With the recent US Food and Drug in the operation of cases where other drugs Administration (FDA) approval of a once-daily extended- seem to offer no relief from pain. Unlike mor - release formulation of the drug (Palladone, Purdue phine, there are no pleasurable sensations to its Pharma LP, Stamford, CT), hydromorphone joins mor - use, however, and if the doctors reckon correctly phine, oxycodone, and fentanyl as the only extended- its use may go far toward curing addicts of the release opioids available on the United States market. morphine habit.” Here, we review the history, pharmacokinetics, and other relevant issues concerning this invaluable opioid, and Montgomery (AL) Advertiser , Dec. 18, 1932 also discuss the role of the new formulation in the man - agement of chronic pain. From 1929 to 1939, the National Research Council’s Committee on Drug Addiction conducted exhaustive history research on the morphine molecule and its analogs, pro - ducing more than 150 semisynthetic and more than 300 Hydromorphone [also Dilaudid (Knoll Laboratories, synthetic compounds, of which more than 30 were tested Mount Olive, NJ), dihydromorphinone, dihydromorphe - clinically. -

“Biosynthesis of Morphine in Mammals”
“Biosynthesis of Morphine in Mammals” D i s s e r t a t i o n zur Erlangung des akademischen Grades Doctor rerum naturalium (Dr. rer. nat.) vorgelegt der Naturwissenschaftlichen Fakultät I Biowissenschaften der Martin-Luther-Universität Halle-Wittenberg von Frau Nadja Grobe geb. am 21.08.1981 in Querfurt Gutachter /in 1. 2. 3. Halle (Saale), Table of Contents I INTRODUCTION ........................................................................................................1 II MATERIAL & METHODS ........................................................................................ 10 1 Animal Tissue ....................................................................................................... 10 2 Chemicals and Enzymes ....................................................................................... 10 3 Bacteria and Vectors ............................................................................................ 10 4 Instruments ........................................................................................................... 11 5 Synthesis ................................................................................................................ 12 5.1 Preparation of DOPAL from Epinephrine (according to DUNCAN 1975) ................. 12 5.2 Synthesis of (R)-Norlaudanosoline*HBr ................................................................. 12 5.3 Synthesis of [7D]-Salutaridinol and [7D]-epi-Salutaridinol ..................................... 13 6 Application Experiments ..................................................................................... -

Morphine Julie Brousseau a Thesis Submitted
Synthesis of Carbocycles Using Coinage Metal Catalysis and Formal Synthesis of (±)-Morphine Julie Brousseau A thesis submitted in partial fulfillment of the requirements for the Doctorate in Philosophy degree in Chemistry Department of Chemistry and Biomolecular Sciences Faculty of Science University of Ottawa © Julie Brousseau, Ottawa, Canada, 2020 ABSTRACT Coinage metals such as copper, silver and gold have captivated mankind with their desirable qualities and social value. Recently, these metals have peaked the interests of scientists, where organic chemists have used them extensively in the homogenous catalysis of organic transformations. In our laboratory, we exploited their � -Lewis acidic properties to activate alkyne to induce intramolecular cyclization of nucleophilic enol ethers. We discovered that modulating the steric and electronic profiles of the ancillary ligand on the cationic metal complexes allowed for the regioselective control of such reactions. During the exploration of the substrate dependency of these transformations, we discovered that unsubstituted alkynes undergo a 6-endo-dig/acetalization/Prins reaction cascade in the presence of a silver salt such as [(BrettPhos)Ag(MeCN)]SbF6, resulting in the formation of highly strained polycycles. We have demonstrated that the formation of these products is initiated by a selective 6-endo-dig cyclization. Further mechanistic studies suggested that the reaction may occur through silver dual catalysis using deuterium-labelling experiments, however, single activation of the starting material would lead to the same product and thus both mechanisms were proposed. The further reactivity of these interesting polycyclic products was also explored. ii OTIPS R2 O OTIPS R1 [(BrettPhos)Ag(MeCN)]SbF6 (10 mol%) DCM, 60°C, 36h R1 9 Examples O R2 23-95% Yields Via O H/[Ag] R1 [Ag] O R2 Total synthesis of natural products is often referred to as an art, as it defines the boundaries of organic chemistry. -

Morphinone Reductase for the Preparation Of
Europäisches Patentamt *EP000649465B1* (19) European Patent Office Office européen des brevets (11) EP 0 649 465 B1 (12) EUROPEAN PATENT SPECIFICATION (45) Date of publication and mention (51) Int Cl.7: C12N 9/02, C12P 17/18, of the grant of the patent: C12Q 1/32 24.07.2002 Bulletin 2002/30 (86) International application number: (21) Application number: 93913236.1 PCT/GB93/01129 (22) Date of filing: 28.05.1993 (87) International publication number: WO 94/00565 (06.01.1994 Gazette 1994/02) (54) MORPHINONE REDUCTASE FOR THE PREPARATION OF HYDROMORPHONE AND HYDROCODONE MORPHINONE REDUKTASE ZUR HERSTELLUNG VON HYDROMORPHONE UND HYDROCODONE MORPHINONE REDUCTASE DESTINEE A LA PREPARATION D’HYDROMORPHONE ET D’HYDROCODONE (84) Designated Contracting States: (74) Representative: Perry, Robert Edward AT BE CH DE DK ES FR GB IE IT LI NL PT SE GILL JENNINGS & EVERY Broadgate House (30) Priority: 25.06.1992 GB 9213524 7 Eldon Street London EC2M 7LH (GB) (43) Date of publication of application: 26.04.1995 Bulletin 1995/17 (56) References cited: WO-A-90/13634 (73) Proprietor: MACFARLAN SMITH LIMITED Edinburgh EH11 2QA (GB) • THE BIOCHEMICAL JOURNAL vol. 274, no. 3, 15 March 1991, pages 875 - 880 NEIL C. BRUCE ET (72) Inventors: AL. ’Microbial degradation of the morphine • HAILES, Anne, Maria 59 Lower Road alkaloids. Purification and characterization of Kent DA8 1AY (GB) morphine dehydrogenase from Pseudomonas • FRENCH, Christopher, Edward putida M10’ Palmerston North (NZ) • BRUCE, Neil, Charles Cambridge CB2 1ND (GB) Note: Within nine months from the publication of the mention of the grant of the European patent, any person may give notice to the European Patent Office of opposition to the European patent granted. -

Ancestral Class-Promiscuity As a Driver of Functional Diversity in the BAHD
bioRxiv preprint doi: https://doi.org/10.1101/2020.11.18.385815; this version posted November 20, 2020. The copyright holder for this preprint (which was not certified by peer review) is the author/funder. All rights reserved. No reuse allowed without permission. 1 Ancestral class-promiscuity as a driver of functional diversity in the 2 BAHD acyltransferase family in plants 3 Lars H. Kruse1, Austin T. Weigle3, Jesús Martínez-Gómez1,2, Jason D. Chobirko1,5, Jason 4 E. Schaffer6, Alexandra A. Bennett1,7, Chelsea D. Specht1,2, Joseph M. Jez6, Diwakar 5 Shukla4, Gaurav D. Moghe1* 6 Footnotes: 7 1 Plant Biology Section, School of Integrative Plant Sciences, Cornell University, Ithaca, 8 NY, 14853, USA 9 2 L.H. Bailey Hortorium, Cornell University, Ithaca, NY, 14853, USA 10 3 Department of Chemistry, University of Illinois at Urbana-Champaign, Urbana, IL, 61801, 11 USA 12 4 Department of Chemical and Biomolecular Engineering, University of Illinois at Urbana- 13 Champaign, Urbana, IL, 61801, USA 14 5 Present address: Department of Molecular Biology and Genetics, Cornell University, 15 Ithaca, NY, 14853, USA 16 6 Department of Biology, Washington University in St. Louis, St. Louis, MO, 63130, USA 17 7 Present address: Institute of Analytical Chemistry, Universität für Bodenkultur Wien, 18 Vienna, 1190, Austria 19 20 * Corresponding author: [email protected] 21 1 bioRxiv preprint doi: https://doi.org/10.1101/2020.11.18.385815; this version posted November 20, 2020. The copyright holder for this preprint (which was not certified by peer review) is the author/funder. All rights reserved. No reuse allowed without permission. -

Microbial Degradation of the Morphine Alkaloids Purification and Characterization of Morphine Dehydrogenase from Pseudomonas Putida M10
Biochem. J. (1991) 274, 875-880 (Printed in Great Britain) 875 Microbial degradation of the morphine alkaloids Purification and characterization of morphine dehydrogenase from Pseudomonas putida M10 Neil C. BRUCE,* Clare J. WILMOT, Keith N. JORDAN, Lauren D. Gray STEPHENS and Christopher R. LOWE Institute of Biotechnology, University of Cambridge, Tennis Court Road, Cambridge CB2 1QT, U.K. The NADP+-dependent morphine dehydrogenase that catalyses the oxidation of morphine to morphinone was detected in glucose-grown cells of Pseudomonas putida M 10. A rapid and reliable purification procedure involving two consecutive affinity chromatography steps on immobilized dyes was developed for purifying the enzyme 1216-fold to electrophoretic homogeneity from P. putida M 10. Morphine dehydrogenase was found to be a monomer of Mr 32000 and highly specific with regard to substrates, oxidizing only the C-6 hydroxy group of morphine and codeine. The pH optimum of morphine dehydrogenase was 9.5, and at pH 6.5 in the presence of NADPH the enzyme catalyses the reduction of codeinone to codeine. The Km values for morphine and codeine were 0.46 mm and 0.044 mm respectively. The enzyme was inhibited by thiol-blocking reagents and the metal-complexing reagents 1, 10-phenanthroline and 2,2'-dipyridyl, suggesting that a metal centre may be necessary for activity of the enzyme. INTRODUCTION EXPERIMENTAL The morphine alkaloids have attracted considerable attention Materials owing to their analgaesic properties and, consequently, much Mimetic Orange 3 A6XL and Mimetic Red A6XL were effort in the past has been directed at the production of new obtained from Affinity Chromatography Ltd., Freeport, morphine alkaloids by micro-organisms (lizuka et al., 1960, Ballasalla, Isle of Man, U.K. -

Feasibility Study on Opium Licensing in Afghanistan
FEASIBILITY STUDY ON OPIUM LICENSING IN AFGHANISTAN FOR THE PRODUCTION OF MORPHINE AND OTHER ESSENTIAL MEDICINES ﻣﻄﺎﻟﻌﻪ اﻣﮑﺎﻧﺎت در ﻣﻮرد ﺟﻮاز دهﯽ ﺗﺮﻳﺎک در اﻓﻐﺎﻧﺴﺘﺎن ﺑﺮای ﺗﻮﻟﻴﺪ ﻣﻮرﻓﻴﻦ و ادوﻳﻪ ﺟﺎت ﺿﺮوری دﻳﮕﺮ Initial Findings – September 2005 Kabul, Afghanistan The British Institute of International and Comparative Law Hugo Warner • University of Calgary Peter Facchini - Jill Hagel University of Ghent Brice De Ruyver - Laurens van Puyenbroeck University of Kabul Abdul Aziz Ali Ahmad - Osman Babury Cheragh Ali Cheragh - Mohammad Yasin Mohsini University of Lisbon Vitalino Canas - Nuno Aureliano • Shruti Patel • University of Toronto Benedikt Fischer Todd Culbert - Juergen Rehm • Wageningen University Jules Bos - Suzanne Pegge • Ali Wardak • The Senlis Council Gabrielle Archer - Juan Arjona - Luke Bryant Marc Das Gupta - Furkat Elmirzaev - Guillaume Fournier Jane Francis - Thalia Ioannidou - Ernestien Jensema Manna Kamio Badiella - Jorrit Kamminga - Fabrice Pothier Emmanuel Reinert - David Spivack - Daniel Werb FEASIBILITY STUDY ON OPIUM LICENSING IN AFGHANISTAN FOR THE PRODUCTION OF MORPHINE AND OTHER ESSENTIAL MEDICINES Initial Findings – September 2005 Kabul, Afghanistan Study Commissioned by The Senlis Council Study Edited and coordinated by David Spivack Editorial team: Juan Arjona, Jane Francis, Thalia Ioannidou, Ernestien Jensema, Manna Kamio Badiella, Fabrice Pothier. Published 2005 by MF Publishing Ltd 17 Queen Anne’s Gate, London SW1H 9BU, UK ISBN: 0-9550798-2-9 Printed and bound in Afghanistan by Jehoon; Printing Press Other publications -

102 4. Biosynthesis of Natural Products Derived from Shikimic Acid
102 4. Biosynthesis of Natural Products Derived from Shikimic Acid 4.1. Phenyl-Propanoid Natural Products (C6-C3) The biosynthesis of the aromatic amino acids occurs through the shikimic acid pathway, which is found in plants and microorganisms (but not in animals). We (humans) require these amino acids in our diet, since we are unable to produce them. For this reason, molecules that can inhibit enzymes on the shikimate pathway are potentially useful as antibiotics or herbicides, since they should not be toxic for humans. COO COO NH R = H Phenylalanine 3 R = OH Tyrosine R NH3 N Tryptophan H The aromatic amino acids also serve as starting materials for the biosynthesis of many interesting natural products. Here we will focus on the so-called phenyl-propanoide (C6-C3) natural products, e.g.: OH OH OH HO O HO OH HO O Chalcone OH O a Flavone OH O OH O a Flavonone OH OH Ar RO O O O HO O O OH O OR OH Anthocyanine OH O a Flavonol Podophyllotoxin MeO OMe OMe OH COOH Cinnamyl alcohol HO O O Cinnamic acid OH (Zimtsäure) Umbellierfone OH a Coumarin) MeO OH O COOH HO Polymerization OH Wood OH HO OH O OH MeO OMe Shikimic acid O HO 4.2. Shikimic acid biosynthesis The shikimic acid pathway starts in carbohydrate metabolism. Given the great social and industrial significance of this pathway, the enzymes have been intensively investigated. Here we will focus on the mechanisms of action of several key enzymes in the pathway. The following Scheme shows the pathway to shikimic acid: 103 COO- COO- Phosphoenolpyruvate HO COO- 2- O O3P-O 2- O3P-O DHQ-Synthase -
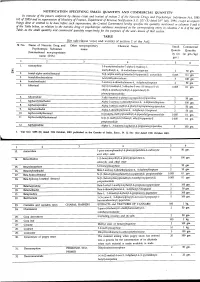
NOTIFICATION SPECIFYING SMALL QUANTITY and COMMERCIAL QUANTITYI Ly of the Pozoersconferred (Xxiiiil
NOTIFICATION SPECIFYING SMALL QUANTITY AND COMMERCIAL QUANTITYI ly of the pozoersconferred (xxiiiil . - lleyise by clansesfuiia) and of section2 of the Nnrcotic Drrtgsnnd pnlchotropic SrtbstancesAct, 19g5 (61 of 1985)and in supersessionof Ministry of Ftnnnce, (D Departmentof'Reuenue Noiification s.o.527 aotra i'6tt,1uli, tssi, exceptas respects things doneor omitted to be done .beforesuch supersession,the Cential Gooernmenihereby spectfiesthe qtnntitrl ,nlnt'ionedin coltnnnsS and 6 of the Tablebelow, in relationto.the narcoticdrug or psychotropicsubstnnce mentioned.ii tlr', ,1urrponding entnl in columns2 to 4 of thesaid Table,as the small quantitrl nnd commercialquantity respectiailyfor the purposesof the said clnuse:sof ttrit seciion. TABLE [Seesub-clause vii(a) and xxiii(a) of section 2 of the Act] Sl No. Name of Narcotic Drug and Other non-proprietary Chemical Name Small Commercial Psychotropic Substance name Quanti- Quantity (Intemational non-proprietary ity (in (in gm./kg.) name (INN) Acetorphine 3-0-acetyltetrahydro-7-alpha-(l-hydroxy-l- methylbutyt)-o, l4-endoetheno-onpavine $ 50 9.. 1\) Acetyl-alpha-methylfen N-[-(alpha-methylphenethyl)-4-piperidy]l acetanilide 0.1 g-. J. Acetyldihydrocodeine 100 4. Acetylmethadol 3-acetoxy-6-dimethylamino-4, 4 lheptane 50 gm. 5. Alfentanil -ethyl-4, N-[1-[2-( 5-dihydro-S-oxo-lH-tetrazol-t-yt) 01 gm. ethyll-4-(methoxymethyl)-4-piperidinyll -N- ylpropanamide Allyprodine 3-allyl Jmethyl-4-phenyl-4 Alpha-3-acetoxy-6-dimethylamino-4,Ld lheptane 100 gm Alpha-3-ethyl-l-methyl-4-phenyl-4-propionox 50 gm. 9.7' AlphamethadolArPnametnaool Alpha-6-dimethylamino-4, 4-diphenyl-3-heptanol 2 S0 gm. 10. Alphu-*"thylf"ntur,yl 11. -

Metabolic Engineering of Escherichia Coli for Natural Product Biosynthesis
Trends in Biotechnology Special Issue: Metabolic Engineering Review Metabolic Engineering of Escherichia coli for Natural Product Biosynthesis Dongsoo Yang,1,4 Seon Young Park,1,4 Yae Seul Park,1 Hyunmin Eun,1 and Sang Yup Lee1,2,3,∗ Natural products are widely employed in our daily lives as food additives, Highlights pharmaceuticals, nutraceuticals, and cosmetic ingredients, among others. E. coli has emerged as a prominent host However, their supply has often been limited because of low-yield extraction for natural product biosynthesis. from natural resources such as plants. To overcome this problem, metabolically Escherichia coli Improved enzymes with higher activity, engineered has emerged as a cell factory for natural product altered substrate specificity, and product biosynthesis because of many advantages including the availability of well- selectivity can be obtained by structure- established tools and strategies for metabolic engineering and high cell density based or computer simulation-based culture, in addition to its high growth rate. We review state-of-the-art metabolic protein engineering. E. coli engineering strategies for enhanced production of natural products in , Balancing the expression levels of genes together with representative examples. Future challenges and prospects of or pathway modules is effective in natural product biosynthesis by engineered E. coli are also discussed. increasing the metabolic flux towards target compounds. E. coli as a Cell Factory for Natural Product Biosynthesis System-wide analysis of metabolic Natural products have been widely used in food and medicine in human history. Many of these networks, omics analysis, adaptive natural products have been developed as pharmaceuticals or employed as structural backbones laboratory evolution, and biosensor- based screening can further increase for the development of new drugs [1], and also as food and cosmetic ingredients. -

Drug Discovery and Development with Plant-Derived Compounds
Progress in Drug Research Founded by Ernst Jucker Series Editors Prof. Dr. Paul L. Herrling Alex Matter, M.D., Director Novartis International AG Novartis Institute for Tropical Diseases CH-4002 Basel 10 Biopolis Road, #05-01 Chromos Switzerland Singapore 138670 Singapore Progress in Drug Research Natural Compounds as Drugs Volume I Vol. 65 Edited by Frank Petersen and René Amstutz Birkhäuser Basel • Boston • Berlin Editors Frank Petersen René Amstutz Novartis Pharma AG Lichtstrasse 35 4056 Basel Switzerland Library of Congress Control Number: 2007934728 Bibliographic information published by Die Deutsche Bibliothek Die Deutsche Bibliothek lists this publication in the Deutsche Nationalbibliografie; detailed bibliographic data is available in the internet at http://dnb.ddb.de ISBN 978-3-7643-8098-4 Birkhäuser Verlag AG, Basel – Boston – Berlin The publisher and editor can give no guarantee for the information on drug dosage and administration contained in this publication. The respective user must check its accuracy by consulting other sources of reference in each individual case. The use of registered names, trademarks etc. in this publication, even if not identified as such, does not imply that they are exempt from the relevant protective laws and regulations or free for general use. This work is subject to copyright. All rights are reserved, whether the whole or part of the material is concerned, specifically the rights of translation, reprinting, re-use of illustrations, recitation, broad- casting, reproduction on microfilms or in other ways, and storage in data banks. For any kind of use, permission of the copyright owner must be obtained. © 2008 Birkhäuser Verlag AG Basel · Boston · Berlin P.O.