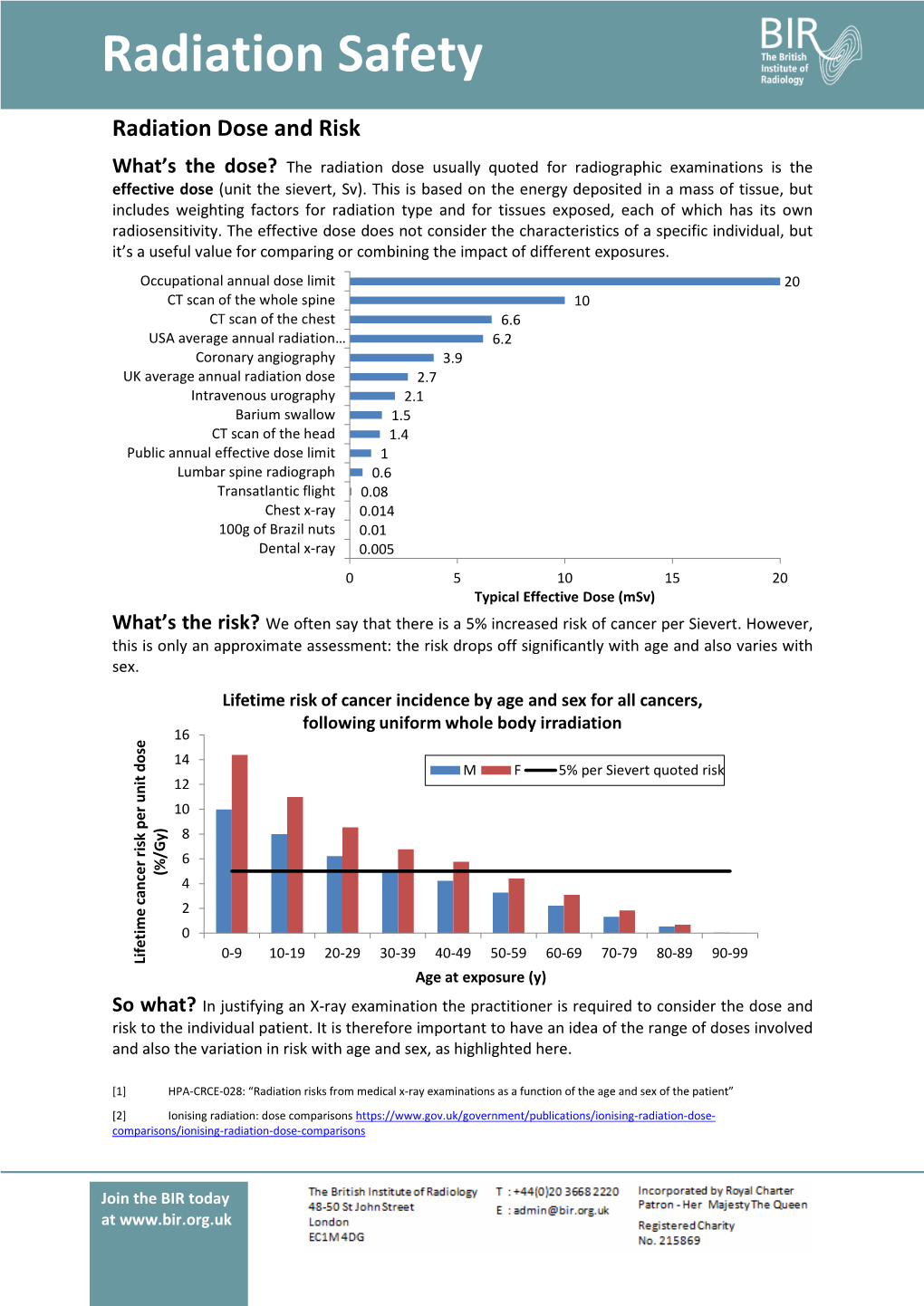
Radiation Safety
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Industrial Radiography
RADIATION PROTECTION OF WORKERS Industrial Radiography RADIATION AND RADIOGRAPHS RADIOACTIVE SOURCES PROCEDURES RADIOGRAPHERS DO follow the procedures. Ionizing radiation can pen- Materials of higher den Sealed sources are small þ Safe storage Precautions þ DO use the appropriate equipment, including collimators. in size and contain material etrate objects and create sity absorb more radiation. þ DO confi rm that there are no other people working in the images on photographic The metal components are which emits penetrating area of radiography. fi lm. The technique is revealed inside this tele radiation continuously. Radioactive sources should be kept in a secure, fi re þ DO use clear working signs and signals. called radiography and phone because they have Special containers made þ DO set up the controlled area and the necessary barriers. the processed fi lms are absorbed more radiation of dense metal shielding resistant and adequately shielded storage location þ DO confi rm the location of the source, or that X rays are called radiographs. than the surrounding plastic. are necessary to store, not being generated, by use of a survey meter. when not in use, and should move and manipulate these þ DO secure and store the source or X ray machine when sources. Due to their small be kept separate from other not in use. materials. The storage loca- size and manoeuvrability, Portable and mobile radiographic þ DO wear your personal dosimeter. sealed sources can be containers. ~ tion for X ray machines that used in confined spaces. are not in use is not required to be shielded. OTHER WORKERS Iridium-192 is a common radioactive source used þ DO observe the access restrictions, however remote it may in gamma radiography. -

Sievert Roofing Products Catalog
Heating tools for professionals Distributed by: BEST MATERIALS LLC Ph: 1-800-474-7570, 1-602-272-8128 Fax: 1-602-272-8014 Email: [email protected] www.bestmaterials.com Roofing Catalog Sievert Industries, Inc. Edition 9 Sievert Industries, Inc. In 1882, the Swedish inventor, Carl Richard Nyberg The Leader in Torch worked in his kitchen to design a revolutionary product, Technolog since1882 a vaporization torch for petrol. During the same year, he obtained a patent for his product which he called a “blow lamp”. This “blow lamp,” or torch, was distributed throughout the world with the help of the famous industrialist, Max Sievert. Carrying on Max Sievert’s work ethic, Sievert Industries, Inc. continually strives to be the leader in the North and South American roofing market since our entrance in 1996. Our goal is to provide our valued customers with quality service, competitive pricing, and the highest level of dependable roofing equipment available. Table of Contents Featured Products.. 7 Sievert Safety.. 8 - 9 Sievert Turboroofer Torch Kits. 10 Sievert Turboroofer Multi-Piece Torch Kits.. 11 Sievert Turboroofer Torch Kit Accessories. 12 Sievert Promatic Torches and Kits.. 13 Sievert Promatic Repair Kits. .. 14 Sievert Promatic Torch Kit Accessories . .15 Sievert Granule Embedders, Sievert Industrial Steel Roller and Sievert Quality Hand Irons . .16 Sievert ES Soldering Iron Kits. 17 Sievert SIK Premium Soldering Iron Kits.. 18 Sievert LSK Premium Basic Soldering Iron Kits.. .19 Sievert ES, SIK and LSK Soldering Iron Kit Accessories.. .20 Sievert Heavy Duty Electronic Hot Air Guns and Accessories. .21 Sievert TW 5000 Hot-Air Automatic Welding Machine and Accessories. -

What Are Health Risks from Ionising Radiation?
What are health risks from Ionising Radiation? It has been known for many years that large doses of ionising radiation, very every 100 persons exposed to a short-term dose of 1000 mSv (ie. if the much larger than background levels, can cause a measurable increase in normal incidence of fatal cancer were 25%, this dose would increase it to cancers and leukemias (‘cancer of the blood’) after some years delay. It must 30%).If doses greater than 1000 mSv occur over a long period they are also be assumed, because of experiments on plants and animals, that ionising less likely to have early health effects but they create a definite risk that radiation can also cause genetic mutations that affect future generations, cancer will develop many years later. although there has been no evidence of radiation-induced mutation in Higher accumulated doses of radiation might produce a cancer which humans. At very high levels, radiation can cause sickness and death within would only be observed several – up to twenty – years after the radiation weeks of exposure. exposure. This delay makes it impossible to say with any certainty which The degree of damage caused by radiation depends on many factors – of many possible agents were the cause of a particular cancer. In western dose, dose rate, type of radiation, the part of the body exposed, age and countries, about a quarter of people die from cancers, with smoking, health, for example. Embryos including the human fetus are particularly dietary factors, genetic factors and strong sunlight being among the sensitive to radiation damage. -

Internal and External Exposure Exposure Routes 2.1
Exposure Routes Internal and External Exposure Exposure Routes 2.1 External exposure Internal exposure Body surface From outer space contamination and the sun Inhalation Suspended matters Food and drink consumption From a radiation Lungs generator Radio‐ pharmaceuticals Wound Buildings Ground Radiation coming from outside the body Radiation emitted within the body Radioactive The body is equally exposed to radiation in both cases. materials "Radiation exposure" refers to the situation where the body is in the presence of radiation. There are two types of radiation exposure, "internal exposure" and "external exposure." External exposure means to receive radiation that comes from radioactive materials existing on the ground, suspended in the air, or attached to clothes or the surface of the body (p.25 of Vol. 1, "External Exposure and Skin"). Conversely, internal exposure is caused (i) when a person has a meal and takes in radioactive materials in the food or drink (ingestion); (ii) when a person breathes in radioactive materials in the air (inhalation); (iii) when radioactive materials are absorbed through the skin (percutaneous absorption); (iv) when radioactive materials enter the body from a wound (wound contamination); and (v) when radiopharmaceuticals containing radioactive materials are administered for the purpose of medical treatment. Once radioactive materials enter the body, the body will continue to be exposed to radiation until the radioactive materials are excreted in the urine or feces (biological half-life) or as the radioactivity weakens over time (p.26 of Vol. 1, "Internal Exposure"). The difference between internal exposure and external exposure lies in whether the source that emits radiation is inside or outside the body. -

Light Radiation
Radiation and Radioactivity Radiation, Radioactivity and Radioactive Materials Radiation and Radioactivity 1.1 Lightbulb = Has the ability to emit light Light Lumen (lm) or Watt (W) Lux (lx) ▶Unit of light bulb brightness ▶Unit of brightness Radioactive materials = Have the ability to emit radiation (radioactivity) Radiation Becquerel (Bq) Conversion Sievert (Sv) ▶Unit of radiation exposure ▶ Unit of radioactivity factor dose that a person receives *Sievert is associated with radiation effects. Radiation, radioactivity and radioactive materials are outlined below. A light bulb, an object familiar to everyone, has the ability to emit light. Light bulb brightness is expressed in the unit of "Lumens" or "Watts." People receive the light and feel the brightness. The unit in this case is "Lux." The units related to radiation, such as becquerel and sievert, which we often hear about lately, also have a similar relation to the above. For example, when a rock emits radiation, this rock is called a "radioactive material" (p.3 of Vol. 1, "Units of Radiation and Radioactivity"). Radioactive materials emit radiation, and this ability is called "radioactivity." In this case, it is expressed as "This rock has radioactivity" or "This rock emits radiation." This ability of emitting radiation is expressed in the unit of "Becquerel (Bq)." "Sievert (Sv)" is used as the unit of the radiation exposure dose necessary to know the effect of radiation to which a person is exposed. There is a special conversion factor to calculate "Sv" from "Bq." Higher radioactivity (value expressed in becquerels) means that the relevant radioactive material emits more radiation, but radiation exposure dose (value expressed in sieverts) varies depending on the distance between the radioactive material and the person exposed thereto. -

Q: What's the Difference Between Roentgen, Rad and Rem Radiation Measurements?
www.JICReadiness.com Q: What's the Difference Between Roentgen, Rad and Rem Radiation Measurements? A: Since nuclear radiation affects people, we must be able to measure its presence. We also need to relate the amount of radiation received by the body to its physiological effects. Two terms used to relate the amount of radiation received by the body are exposure and dose. When you are exposed to radiation, your body absorbs a dose of radiation. As in most measurement quantities, certain units are used to properly express the measurement. For radiation measurements they are... Roentgen: The roentgen measures the energy produced by gamma radiation in a cubic centimeter of air. It is usually abbreviated with the capital letter "R". A milliroentgen, or "mR", is equal to one one-thousandth of a roentgen. An exposure of 50 roentgens would be written "50 R". Rad: Or, Radiation Absorbed Dose recognizes that different materials that receive the same exposure may not absorb the same amount of energy. A rad measures the amount of radiation energy transferred to some mass of material, typically humans. One roentgen of gamma radiation exposure results in about one rad of absorbed dose. Rem: Or, Roentgen Equivalent Man is a unit that relates the dose of any radiation to the biological effect of that dose. To relate the absorbed dose of specific types of radiation to their biological effect, a "quality factor" must be multiplied by the dose in rad, which then shows the dose in rems. For gamma rays and beta particles, 1 rad of exposure results in 1 rem of dose. -

Radiation Safety: New International Standards
FEATURES Radiation safety: New international standards The forthcoming International Basic Safety Standards for Protection Against Ionizing Radiation and for the Safety of Radiation Sources are the product of unprecedented co-operation by Abel J. B*y the end of the 1980s, a vast amount of new This article highlights an important result of Gonzalez information had accumulated to prompt a new this work for the international harmonization of look at the standards governing protection radiation safety: specifically, it presents an over- against exposures to ionizing radiation and the view of the forthcoming International Basic safety of radiation sources. Safety Standards for Protection Against Ionizing First and foremost, a re-evaluation of the Radiation and for the Safety of Radiation Sour- radioepidemiological findings from Hiroshima ces — the so-called BSS. They have been jointly and Nagasaki suggested that exposure to low- developed by six organizations — the Food and level radiation was riskier than previously es- Agriculture Organization of the United Nations timated. (FAO), the International Atomic Energy Agency Other developments — notably the nuclear (IAEA), the International Labour Organization accidents at Three Mile Island in 1979 and at (ILO), the Nuclear Energy Agency of the Or- Chernobyl in 1986 with its unprecedented ganization for Economic Co-operation and transboundary contamination — had a great ef- Development (NEA/OECD), the Pan American fect on the public perception of the potential Health Organization (PAHO), and the World danger from radiation exposure. Accidents with Health Organization (WHO). radiation sources used in medicine and industry also have attracted widespread public attention: Cuidad Juarez (Mexico), Mohamadia (Moroc- The framework for harmonization co), Goiania (Brazil), San Salvador (El Sal- vador), and Zaragoza (Spain) are names that ap- In 1991, within the framework of the Inter- peared in the news after people were injured in agency Committee on Radiation Safety, the six radiation accidents. -

Heating Tools for Professionals Product Catalogue
Heating tools for professionals Product Catalogue Swedish design and quality since 1882 In your hand you hold Sievert’s whole product range. But behind all the product pictures and article numbers there is a lot more. This unseen Sievert represents more than hund- redthirty years of technical progress - decades of technical support and discussions with users - years of providing thoughtful service - days of unceasing effort to find new possibilities. Our stated goal is both simple and straightforward: to develop, manufacture and supply innovative and high- quality tools and tool systems for all types of soldering and heating applications. This statement is not just a definition of our product range – it is also a promise. A promise that we will al- ways be one step ahead and that we will always lis- ten to you – our customer – and involve ourselves in The concept is over a hundred your business. A promise that we will be close to hand years old but the flame still wherever in the world you may be. A promise that we will be the working partner that you need, helping you burns as brightly today achieve the greatest possible success in your business when you use our products and services. Whatever the jobs you may have on today. Whatever the jobs you may Because Carl Richard Nyberg was a specialist in have in the future. soldering, he knew all too well that the soldering technology available in the 1880´s left a lot to be desired. We have been in business for more than 130 years, and today our products lead the world. -

Background Radiation Fact Sheet
Health Physics Society Fact Sheet Adopted: June 2012 Revised: June 2015 Revised: September 2020 Health Physics Society Specialists in Radiation Safety Common Sources of Radiation Background radiation surrounds us at all times—it is everywhere. Since the earth was formed and life developed, all life on earth has been exposed to ionizing radiation1. This fact sheet addresses the baseline sources of background ionizing radiation and other sources of radiation to which we are commonly exposed on a daily basis. Sources of Radiation Background radiation is emitted from both natural and human-made radionuclides, or radioactive atoms. Some naturally occurring radiation comes from the atmosphere as a result of radiation from outer space, some comes from the earth, and some is even in our bodies as a result of radionuclides in the food and water we ingest and the air we breathe. Additionally, some human-made radiation sources include consumer products, nuclear and coal- burning power plants, and medical uses such as nuclear medicine and computed tomography (CT). Annually, a US citizen is exposed to an average radiation dose of 6.2 millisieverts (mSv) Fig. 1 depicts the typical distribution of exposure from all sources of radiation. As can be seen, natural background radiation, also called “ubiquitous” since it is around us at all times, is the largest source of radiation exposure to humans (50% or about 3.1 mSv). Fig. 1. Source distribution for all radiation dose—percent contribution of various sources of exposure to the total dose per individual in the US Radionuclides in the Body population. Adapted from Ionizing Radiation Exposure of the Population of the United States (NCRP 2009). -

Fact Sheet – Natural Background Radiation
Fact sheet Natural Background Radiation November 2020 Quick Facts Radiation is energy in motion, in the form of waves or streams of particles. Radiation has always been Radiation has always been present and is all around us in many forms. present and is all around us in When people hear the word radiation, they often think many natural forms. Life has of atomic energy, nuclear power and radioactivity, but evolved in a world with radiation has many different forms and comes from significant levels of ionizing many other sources. Sound and visible light are radiation. familiar forms of radiation; other types include Many radioisotopes are ultraviolet radiation (that produces a suntan), infrared naturally occurring, and radiation (a form of heat energy), and radio and originated during the formation television signals. These are examples of non-ionizing of the solar system and through radiation. the interaction of cosmic rays Ionizing radiation has the ability to knock electrons out with molecules in the of orbit around atoms, upsetting the electron/proton atmosphere. Tritium is an balance and potentially damaging cells. Examples example of a radioisotope include alpha, beta, gamma and neutron radiation and formed by this interaction. x-rays. Radioisotopes such as Life has evolved in a world with significant levels of polonium-210, carbon-14 and ionizing radiation and our bodies have adapted to it. potassium-40 naturally occur within the human body. Potassium-40 is present in many common foods including red meats, white potatoes, carrots, bananas, lima beans and Brazil nuts. The annual average effective dose from natural background radiation is approximately 1.8 millisieverts (mSv) in Canada and 2.4 mSv worldwide. -

Bq = Becquerel Gy = Gray (Sv = Sievert)
Louis Harold Gray He is honored by call- ing the physical dose Bq = becquerel unit "gray*" – abbrevi- Gy = gray ated Gy (Sv = sievert) Photo from 1957 Chapter 5 Activity and Dose The activity of a radioactive source When an atom disintegrates, radiation is emitted. If the rate of disintegrations is large, the radioactive source is considered to have a high activity. The unit for the activity of a radioactive source was named after Becquerel (abbreviated Bq) and is defined as: 1 Bq = 1 disintegration per sec. In a number of countries, the old unit, the curie (abbreviated Ci and named after Marie and Pierre Curie) is still used. The curie-unit was defined as the activity in one gram of radium. The number of disintegrations per second in one gram of radium is 37 billion. The relation between the curie and the becquerel is given by: 1 Ci = 3.7 • 1010 Bq The accepted practice is to give the activity of a radioactive source in becquerel. This is because Bq is the unit chosen for the system of international units (SI-units). But one problem is that the numbers in becquerel are always very large. Consequently the activity is given in kilo (103), mega (106), giga (109)and tera (1012) becquerel. If a source is given in curies the number is small. For example; when talking about radioactivity in food products, 3,700 Bq per kilogram of meat is a large number and consequently considered to be dangerous. If however, the same activity is given in Ci, it is only 0.0000001 curie per kilogram – "nothing to worry about?". -

Useful Information
USEFUL INFORMATION Radiation and Radioactivity Radioactivity is a characteristic of some elements that have unstable atomic nuclei, which spontaneously disintegrate or “decay” into atomic nuclei of another isotope or element. The nuclei decay until only a stable, nonradioactive isotope remains. Depending on the isotope, this process can take anywhere from less than a second to billions of years. Atoms that emit radiation are called radionuclides. Radionuclides are unstable isotopes of an element that have the same number of protons but different numbers of neutrons, resulting in different atomic masses. For example, the element hydrogen has two stable isotopes, hydrogen•1 ( 1H) and hydrogen•2 ( 2H) (deuterium), and one radioactive isotope, hydrogen•3 (3H) (tritium). The superscript preceding the element’s symbol identifies the atomic mass, which is the number of protons plus neutrons in the nucleus. Thus, 1H has one proton and no neutrons, 2H has one proton and one neutron, and3H has one proton and two neutrons. When radioactive atoms decay by emitting radiation, the daughter products that result may be either radioactive or stable. Generally, radionuclides with high atomic numbers, such as uranium•238 and plutonium•239, have many generations of radioactive progeny. For example, the radioactive decay of plutonium•239 creates uranium•235, thorium•231, protactinium•231, and so on, through 11 progeny until only the stable isotope lead•207 remains. Radionuclides with lower atomic numbers often have no more than one daughter. For example, strontium•90 has one radioactive daughter, yttrium•90, which finally decays into stable zirconium; cobalt•60 decays directly to stable nickel with no intermediate nuclide.