2015 WHMIS Classification
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Organic Chemistry. T
View Article Online / Journal Homepage / Table of Contents for this issue i. 369 Organic Chemistry. Nature of the Kolbe Electrochemical Synthesis of Hydrocarbons. FH. FICHTERand EDUARDKRUMMENAUHEH (Helvetica Chim. A cia, 1918, 1, 146-166).-The authors discuss the two theories put forward to explain the' mechanism of the Kolbe hydrocarbon synthesis, and draw the conclusion that the oxidation thsory is more in accord with fact than the theory based on the ionic hypothesis. It is shown that peroxides are produced by electrochemical oxidation, on platinum anodes, of solutions of saturated fatty acids and their salts. Since them peroxides may be produced by the action of hydrogen peroxide on aci6 anhydrides, it is to be assumed that the separation of the anhydride is the first step in the hydrocarbon synthesis. The anhydride is then anodic- ally oxidised, with the formation of a very unstable peroxide. The peroxide is then decomposed by the relatively high tempera- ture of the anode, with the formation of the hydrocarbon, thus: R*CO,Na + (RCO),-0 + 0 + (RCO),*O = 0 + R*R+ 2CO,, or a side reaction may also occur, thus: (R*CO),O=O 4 R-CO,*R + CO,. When th? peroxides are decomposed by heat, there is also a considerable amount of methane produced in addition to the expected hydrocarbon. These considerations are adapted to the formation and decom- position of organic peracids. Organic peracids m:by be prepared by the action of hydrogen peroxide on both acids and anhydrides, consequently the formation of these substances electrolytically on the, anode does not necessarily demand the intermediary formation Published on 01 January 1918. -

1 Abietic Acid R Abrasive Silica for Polishing DR Acenaphthene M (LC
1 abietic acid R abrasive silica for polishing DR acenaphthene M (LC) acenaphthene quinone R acenaphthylene R acetal (see 1,1-diethoxyethane) acetaldehyde M (FC) acetaldehyde-d (CH3CDO) R acetaldehyde dimethyl acetal CH acetaldoxime R acetamide M (LC) acetamidinium chloride R acetamidoacrylic acid 2- NB acetamidobenzaldehyde p- R acetamidobenzenesulfonyl chloride 4- R acetamidodeoxythioglucopyranose triacetate 2- -2- -1- -β-D- 3,4,6- AB acetamidomethylthiazole 2- -4- PB acetanilide M (LC) acetazolamide R acetdimethylamide see dimethylacetamide, N,N- acethydrazide R acetic acid M (solv) acetic anhydride M (FC) acetmethylamide see methylacetamide, N- acetoacetamide R acetoacetanilide R acetoacetic acid, lithium salt R acetobromoglucose -α-D- NB acetohydroxamic acid R acetoin R acetol (hydroxyacetone) R acetonaphthalide (α)R acetone M (solv) acetone ,A.R. M (solv) acetone-d6 RM acetone cyanohydrin R acetonedicarboxylic acid ,dimethyl ester R acetonedicarboxylic acid -1,3- R acetone dimethyl acetal see dimethoxypropane 2,2- acetonitrile M (solv) acetonitrile-d3 RM acetonylacetone see hexanedione 2,5- acetonylbenzylhydroxycoumarin (3-(α- -4- R acetophenone M (LC) acetophenone oxime R acetophenone trimethylsilyl enol ether see phenyltrimethylsilyl... acetoxyacetone (oxopropyl acetate 2-) R acetoxybenzoic acid 4- DS acetoxynaphthoic acid 6- -2- R 2 acetylacetaldehyde dimethylacetal R acetylacetone (pentanedione -2,4-) M (C) acetylbenzonitrile p- R acetylbiphenyl 4- see phenylacetophenone, p- acetyl bromide M (FC) acetylbromothiophene 2- -5- -

WO 2014/185925 Al 20 November 2014 (20.11.2014) P O P C T
(12) INTERNATIONAL APPLICATION PUBLISHED UNDER THE PATENT COOPERATION TREATY (PCT) (19) World Intellectual Property Organization International Bureau (10) International Publication Number (43) International Publication Date WO 2014/185925 Al 20 November 2014 (20.11.2014) P O P C T (51) International Patent Classification: AO, AT, AU, AZ, BA, BB, BG, BH, BN, BR, BW, BY, BOW 53/28 (2006.01) BZ, CA, CH, CL, CN, CO, CR, CU, CZ, DE, DK, DM, DO, DZ, EC, EE, EG, ES, FI, GB, GD, GE, GH, GM, GT, (21) International Application Number: HN, HR, HU, ID, IL, IN, IS, JP, KE, KG, KM, KN, KP, PCT/US20 13/04 1503 KR, KZ, LA, LC, LK, LR, LS, LT, LU, LY, MA, MD, (22) International Filing Date: ME, MG, MK, MN, MW, MX, MY, MZ, NA, NG, NI, 17 May 2013 (17.05.2013) NO, NZ, OM, PA, PE, PG, PH, PL, PT, QA, RO, RS, RU, RW, SC, SD, SE, SG, SK, SL, SM, ST, SV, SY, TH, TJ, (25) Filing Language: English TM, TN, TR, TT, TZ, UA, UG, US, UZ, VC, VN, ZA, (26) Publication Language: English ZM, ZW. (71) Applicant: EMPIRE TECHNOLOGY DEVELOP¬ (84) Designated States (unless otherwise indicated, for every MENT LLC [US/US]; 271 1 Centerville Road, Suite 400, kind of regional protection available): ARIPO (BW, GH, Wilmington, Delaware 19808 (US). GM, KE, LR, LS, MW, MZ, NA, RW, SD, SL, SZ, TZ, UG, ZM, ZW), Eurasian (AM, AZ, BY, KG, KZ, RU, TJ, (72) Inventors: PEPPOU, George Charles; 4 Armen Way, TM), European (AL, AT, BE, BG, CH, CY, CZ, DE, DK, Hornsby Heights, New South Wales 2077 (AU). -

Underactive Thyroid
Underactive Thyroid PDF generated using the open source mwlib toolkit. See http://code.pediapress.com/ for more information. PDF generated at: Thu, 21 Jun 2012 14:27:58 UTC Contents Articles Thyroid 1 Hypothyroidism 14 Nutrition 22 B vitamins 47 Vitamin E 53 Iodine 60 Selenium 75 Omega-6 fatty acid 90 Borage 94 Tyrosine 97 Phytotherapy 103 Fucus vesiculosus 107 Commiphora wightii 110 Nori 112 Desiccated thyroid extract 116 References Article Sources and Contributors 121 Image Sources, Licenses and Contributors 124 Article Licenses License 126 Thyroid 1 Thyroid thyroid Thyroid and parathyroid. Latin glandula thyroidea [1] Gray's subject #272 1269 System Endocrine system Precursor Thyroid diverticulum (an extension of endoderm into 2nd Branchial arch) [2] MeSH Thyroid+Gland [3] Dorlands/Elsevier Thyroid gland The thyroid gland or simply, the thyroid /ˈθaɪrɔɪd/, in vertebrate anatomy, is one of the largest endocrine glands. The thyroid gland is found in the neck, below the thyroid cartilage (which forms the laryngeal prominence, or "Adam's apple"). The isthmus (the bridge between the two lobes of the thyroid) is located inferior to the cricoid cartilage. The thyroid gland controls how quickly the body uses energy, makes proteins, and controls how sensitive the body is to other hormones. It participates in these processes by producing thyroid hormones, the principal ones being triiodothyronine (T ) and thyroxine which can sometimes be referred to as tetraiodothyronine (T ). These hormones 3 4 regulate the rate of metabolism and affect the growth and rate of function of many other systems in the body. T and 3 T are synthesized from both iodine and tyrosine. -

Gas Conversion Factor for 300 Series
300GasTable Rec # Gas Symbol GCF Density (g/L) Density (g/L) 25° C / 1 atm 0° C / 1 atm 1 Acetic Acid C2H4F2 0.4155 2.7 2.947 2 Acetic Anhydride C4H6O3 0.258 4.173 4.555 3 Acetone C3H6O 0.3556 2.374 2.591 4 Acetonitryl C2H3N 0.5178 1.678 1.832 5 Acetylene C2H2 0.6255 1.064 1.162 6 Air Air 1.0015 1.185 1.293 7 Allene C3H4 0.4514 1.638 1.787 8 Ammonia NH3 0.7807 0.696 0.76 9 Argon Ar 1.4047 1.633 1.782 10 Arsine AsH3 0.7592 3.186 3.478 11 Benzene C6H6 0.3057 3.193 3.485 12 Boron Trichloride BCl3 0.4421 4.789 5.228 13 Boron Triflouride BF3 0.5431 2.772 3.025 14 Bromine Br2 0.8007 6.532 7.13 15 Bromochlorodifluoromethane CBrClF2 0.3684 6.759 7.378 16 Bromodifluoromethane CHBrF2 0.4644 5.351 5.841 17 Bromotrifluormethane CBrF3 0.3943 6.087 6.644 18 Butane C4H10 0.2622 2.376 2.593 19 Butanol C4H10O 0.2406 3.03 3.307 20 Butene C4H8 0.3056 2.293 2.503 21 Carbon Dioxide CO2 0.7526 1.799 1.964 22 Carbon Disulfide CS2 0.616 3.112 3.397 23 Carbon Monoxide CO 1.0012 1.145 1.25 24 Carbon Tetrachloride CCl4 0.3333 6.287 6.863 25 Carbonyl Sulfide COS 0.668 2.456 2.68 26 Chlorine Cl2 0.8451 2.898 3.163 27 Chlorine Trifluoride ClF3 0.4496 3.779 4.125 28 Chlorobenzene C6H5Cl 0.2614 4.601 5.022 29 Chlorodifluoroethane C2H3ClF2 0.3216 4.108 4.484 30 Chloroform CHCl3 0.4192 4.879 5.326 31 Chloropentafluoroethane C2ClF5 0.2437 6.314 6.892 32 Chloropropane C3H7Cl 0.308 3.21 3.504 33 Cisbutene C4H8 0.3004 2.293 2.503 34 Cyanogen C2N2 0.4924 2.127 2.322 35 Cyanogen Chloride ClCN 0.6486 2.513 2.743 36 Cyclobutane C4H8 0.3562 2.293 2.503 37 Cyclopropane C3H6 0.4562 -

And Perfluoroalkyl Substances (PFAS): Sources, Pathways and Environmental Data
Poly- and perfluoroalkyl substances (PFAS): sources, pathways and environmental data Chief Scientist’s Group report August 2021 We are the Environment Agency. We protect and improve the environment. We help people and wildlife adapt to climate change and reduce its impacts, including flooding, drought, sea level rise and coastal erosion. We improve the quality of our water, land and air by tackling pollution. We work with businesses to help them comply with environmental regulations. A healthy and diverse environment enhances people's lives and contributes to economic growth. We can’t do this alone. We work as part of the Defra group (Department for Environment, Food & Rural Affairs), with the rest of government, local councils, businesses, civil society groups and local communities to create a better place for people and wildlife. Published by: Author: Emma Pemberton Environment Agency Horizon House, Deanery Road, Environment Agency’s Project Manager: Bristol BS1 5AH Mark Sinton www.gov.uk/environment-agency Citation: Environment Agency (2021) Poly- and © Environment Agency 2021 perfluoroalkyl substances (PFAS): sources, pathways and environmental All rights reserved. This document may data. Environment Agency, Bri be reproduced with prior permission of the Environment Agency. Further copies of this report are available from our publications catalogue: www.gov.uk/government/publications or our National Customer Contact Centre: 03708 506 506 Email: research@environment- agency.gov.uk 2 of 110 Research at the Environment Agency Scientific research and analysis underpins everything the Environment Agency does. It helps us to understand and manage the environment effectively. Our own experts work with leading scientific organisations, universities and other parts of the Defra group to bring the best knowledge to bear on the environmental problems that we face now and in the future. -
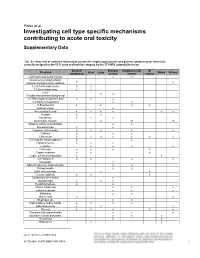
Investigating Cell Type Specific Mechanisms Contributing to Acute Oral Toxicity
Prieto et al.: Investigating cell type specific mechanisms contributing to acute oral toxicity Supplementary Data1 Tab. S1: Overview of collected information on specific target organ/system and general cytotoxicity for chemicals correctly assigned to the CLP acute oral toxicity category by the 3T3 NRU cytotoxicity assay General Nervous Cardiovascular GI Chemical Liver Lung Blood Kidney cytotoxicity system system system (±)-Propranolol hydrochloride x ax (4-ammonio-m-tolyl)ethyl(2- x x hydroxyethyl)ammonium sulphate 1,2,4-Trichlorobenzene x x 1,2-Dichlorobenzene x x 2,4,6- x x Tris(dimethylaminomethyl)phenol 2,4-Dichlorophenoxyacetic acid x x x 5,5-Diphenylhydantoin x x 5-Fluorouracil x x x x Acetophenone x Acetylsalicylic acid x x x x x x Acrolein x x Acrylamide x x x Ammonium chloride x ax ax Atropine sulfate monohydrate x x Benzaldehyde x x Cadmium (III) chloride x x x x x Caffeine x x x Chloroform x x ax x x x x Chloroquine bis(phosphate) x x Chlorpromazine x x x Codeine x x x x Colchicine x x x x Copper sulphate x x x Cupric sulfate pentahydrate x x Cyclosporin A x x x x Diazepam x Diphenhydramine hydrochloride x x Disopyramide x x Ethyl chloroacetate x x Ferrous sulphate x x x Glufosinate-ammonium x Glutethimide x ax x Hexachlorophene x x Lithium Carbonate x x x Lithium sulphate x x x Malathion x x Maleic acid x x Meprobamate x x Orphenadrine hydrochloride x x x x p-Benzoquinone x x x Phenol x x x x x Procainamide hydrochloride x x x Quinidine sulfate dehydrate x x x Resorcinol x x Rifampicin x x doi:10.14573/altex.1805181s2 ALTEX ##(#), SUPPLEMENTARY DATA 1 General Nervous Cardiovascular GI Chemical Liver Lung Blood Kidney cytotoxicity system system system Sodium Cyanate x x sodium oxalate x x x Sodium valproate x bx x x Thioridazine hydrochloride x x Valproic acid x x x x x GI: Gastrointestinal; CLP: Classification, labelling and packaging; NRU: Neutral Red Uptake; a Indirect effect: b chronic effect Tab. -

High Purity Inorganics
High Purity Inorganics www.alfa.com INCLUDING: • Puratronic® High Purity Inorganics • Ultra Dry Anhydrous Materials • REacton® Rare Earth Products www.alfa.com Where Science Meets Service High Purity Inorganics from Alfa Aesar Known worldwide as a leading manufacturer of high purity inorganic compounds, Alfa Aesar produces thousands of distinct materials to exacting standards for research, development and production applications. Custom production and packaging services are part of our regular offering. Our brands are recognized for purity and quality and are backed up by technical and sales teams dedicated to providing the best service. This catalog contains only a selection of our wide range of high purity inorganic materials. Many more products from our full range of over 46,000 items are available in our main catalog or online at www.alfa.com. APPLICATION FOR INORGANICS High Purity Products for Crystal Growth Typically, materials are manufactured to 99.995+% purity levels (metals basis). All materials are manufactured to have suitably low chloride, nitrate, sulfate and water content. Products include: • Lutetium(III) oxide • Niobium(V) oxide • Potassium carbonate • Sodium fluoride • Thulium(III) oxide • Tungsten(VI) oxide About Us GLOBAL INVENTORY The majority of our high purity inorganic compounds and related products are available in research and development quantities from stock. We also supply most products from stock in semi-bulk or bulk quantities. Many are in regular production and are available in bulk for next day shipment. Our experience in manufacturing, sourcing and handling a wide range of products enables us to respond quickly and efficiently to your needs. CUSTOM SYNTHESIS We offer flexible custom manufacturing services with the assurance of quality and confidentiality. -

Chemical Resistance of Plastics
(c) Bürkle GmbH 2010 Important Important information The tables “Chemical resistance of plastics”, “Plastics and their properties” and “Viscosity of liquids" as well as the information about chemical resistance given in the particular product descriptions have been drawn up based on information provided by various raw material manufacturers. These values are based solely on laboratory tests with raw materials. Plastic components produced from these raw materials are frequently subject to influences that cannot be recognized in laboratory tests (temperature, pressure, material stress, effects of chemicals, construction features, etc.). For this reason the values given are only to be regarded as being guidelines. In critical cases it is essential that a test is carried out first. No legal claims can be derived from this information; nor do we accept any liability for it. A knowledge of the chemical and mechanical Copyright This table has been published and updated by Bürkle GmbH, D-79415 Bad Bellingen as a work of reference. This Copyright clause must not be removed. The table may be freely passed on and copied, provided that Extensions, additions and translations If your own experiences with materials and media could be used to extend this table then we would be pleased to receive any additional information. Please send an E-Mail to [email protected]. We would also like to receive translations into other languages. Please visit our website at http://www.buerkle.de from time to Thanks Our special thanks to Franz Kass ([email protected]), who has completed and extended these lists with great enthusiasm and his excellent specialist knowledge. -

Section 2. Hazards Identification OSHA/HCS Status : This Material Is Considered Hazardous by the OSHA Hazard Communication Standard (29 CFR 1910.1200)
SAFETY DATA SHEET Nonflammable Gas Mixture: Carbon Dioxide / Carbonyl Sulfide / Dichlorofluoromethane (R12) / Nitrogen / Sulfur Dioxide / Sulfuryl Fluoride / Tetrafluoromethane (R14) / Thionyl Fluoride Section 1. Identification GHS product identifier : Nonflammable Gas Mixture: Carbon Dioxide / Carbonyl Sulfide / Dichlorofluoromethane (R12) / Nitrogen / Sulfur Dioxide / Sulfuryl Fluoride / Tetrafluoromethane (R14) / Thionyl Fluoride Other means of : Not available. identification Product use : Synthetic/Analytical chemistry. SDS # : 019443 Supplier's details : Airgas USA, LLC and its affiliates 259 North Radnor-Chester Road Suite 100 Radnor, PA 19087-5283 1-610-687-5253 24-hour telephone : 1-866-734-3438 Section 2. Hazards identification OSHA/HCS status : This material is considered hazardous by the OSHA Hazard Communication Standard (29 CFR 1910.1200). Classification of the : GASES UNDER PRESSURE - Compressed gas substance or mixture HAZARDOUS TO THE OZONE LAYER - Category 1 GHS label elements Hazard pictograms : Signal word : Warning Hazard statements : Contains gas under pressure; may explode if heated. May displace oxygen and cause rapid suffocation. Harms public health and the environment by destroying ozone in the upper atmosphere. Precautionary statements General : Read and follow all Safety Data Sheets (SDS’S) before use. Read label before use. Keep out of reach of children. If medical advice is needed, have product container or label at hand. Close valve after each use and when empty. Use equipment rated for cylinder pressure. Do not open valve until connected to equipment prepared for use. Use a back flow preventative device in the piping. Use only equipment of compatible materials of construction. Do not depend on odor to detect presence of gas. Prevention : Not applicable. Response : Not applicable. -

SYNTHESES Volume XIV Editors AARON WOLD JOHN K
INORGANIC SYNTHESES Volume XIV Editors AARON WOLD JOHN K. RUFF Professor of Engineering Associate Professor of Chemistry and Chemistry University of Georgia Brown University, Providence, R.I. Athens, Ca. INORGANIC SYNTHESES Volume XIV McCRAW-HILL BOOK COMPANY New York St. Louis San Francisco Diisseldorf Johannesburg Kualo Lumpur London Mexico Montreal New Delhi Panama Rio de Janeiro Singapore Sydney Toronto INORGANIC SYNTHESES, VOLUME XIV Copyright 0 1973 by McGraw-Hill, Inc. All Rights Rewved. Printed in the United States of America. No part of this publication may be reproduced, stored in a retrieval system, or transmitted, in any form or by any means, electronic, mechanical, photocopying, recording, or otherwise. without the prior written permission of the publisher. Library of Congress Catalog Card Number 39-23015 07-07 1320-0 1234567890 KPKP 76543 To RONALD NYHOLM and DAVID WADSLEY CONTENTS Reface ........................................... xi Notice to Contributors ................................... xiii Chapter One PHOSPHORUS COMPOUNDS ............... 1 1 . Phosphine ....................................... 1 2 . tert-Butyldichlorophosphineand Di-tert-butylchlorophosphinc......... 4 A . tert-Butyldichlorophosphinc ......................... 5 B . Di-tert-butylchlorophosphinc ......................... 6 3. 1.2-Bis(phosphino)ethane ............................. 10 4 . Tctramcthyldiphosphineand Flexible Aliphatic (Dimethylphosphino) Ligands ........................... 14 A . TetramethyldiphosFhine............................. 15 B . -

Inorganic Seminar Abstracts
C 1 « « « • .... * . i - : \ ! -M. • ~ . • ' •» »» IB .< L I B RA FLY OF THE. UN IVERSITY Of 1LLI NOIS 546 1^52-53 Return this book on or before the Latest Date stamped below. University of Illinois Library «r L161— H41 Digitized by the Internet Archive in 2012 with funding from University of Illinois Urbana-Champaign http://archive.org/details/inorganicsemi195253univ INORGANIC SEMINARS 1952 - 1953 TABLE OF CONTENTS 1952 - 1953 Page COMPOUNDS CONTAINING THE SILICON-SULFUR LINKAGE 1 Stanley Kirschner ANALYTICAL PROCEDURES USING ACETIC ACID AS A SOLVENT 5 Donald H . Wilkins THE SOLVENT PHOSPHORYL CHLORIDE, POCl 3 12 S.J. Gill METHODS FOR PREPARATION OF PURE SILICON 17 Alex Beresniewicz IMIDODISULFINAMIDE 21 G.R. Johnston FORCE CONSTANTS IN POLYATOMIC MOLECILES 28 Donn D. Darsow METATHESIS IN LIQUID ARSENIC TRICHLORIDE 32 Harold H. Matsuguma THE RHENI DE OXIDATION STATE 40 Robert L. Rebertus HALOGEN CATIONS 45 L.H. Diamond REACTIONS OF THE NITROSYL ION 50 M.K. Snyder THE OCCURRENCE OF MAXIMUM OXIDATION STATES AMONG THE FLUOROCOMPLEXES OF THE FIRST TRANSITION SERIES 56 D.H. Busch POLY- and METAPHOSPHATES 62 V.D. Aftandilian PRODUCTION OF SILICON CHLORIDES BY ELECTRICAL DISCHARGE AND HIGH TEMPERATURE TECHNIQUES 67 VI. £, Cooley FLUORINE CONTAINING OXYHALIDES OF SULFUR 72 E.H. Grahn PREPARATION AND PROPERTIES OF URANYL CARBONATES 76 Richard *• Rowe THE NATURE OF IODINE SOLUTIONS 80 Ervin c olton SOME REACTIONS OF OZONE 84 Barbara H. Weil ' HYDRAZINE BY ELECTROLYSIS IN LIQUID AMMONIA 89 Robert N. Hammer NAPHTHAZARIN COMPLEXES OF THORIUM AND RARE EARTH METAL IONS 93 Melvin Tecotzky THESIS REPORT 97 Perry Kippur ION-PAIR FORMATION IN ACETIC ACID 101 M.M.