Sampling and Analysis of Soluble Metal Compounds
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Tungstovanadate Heteropoly Complexes. 11. Products of Acidification of V2w&Lg4
TUNGSTOVANADATEHETEROPOLY COMPLEXES Inorganic Chemistry, Vol. 10, No. 12, 1971 2745 Then, assuming that the difference is also reflected in line widths were somewhat broader than those of the Tze,much broader epr lines are expected for the mono- VO(H20)42+spectrum. That observation is compatible chloro species, which would be difficult to detect in the with a decreased re relative to that of the aquo complex, presence of the sharper spectrum of VO(H20)2C12. but a much more detailed investigation of the relaxa- The liquid solution epr spectra for a number of tion processes would be necessary for quantitative HC1 concentrations were also observed in this labora- comparison. tory. The intensity of the spectrum attributed to VO (H~0)4~+appeared to follow qualitatively the rela- Acknowledgments.-We are indebted to Dr. W. tive concentrations indicated in Tables I1 and I11 and Burton Lewis and others for helpful comments during Figure 4. However, no attempt was made to do an discussions of the work reported here. We also wish absolute spin count. In 12 M HC1 solution the (g) to thank Dr. B. B. McInteer and Mr. R. M. Potter of and (A) values obtained corresponded well with the Los Alamos Scientific Laboratory for supplying those reported by Kon and Sharpless and the observed the enriched "'0. CONTRIBUTIONFROM THE DEPARTMENTOF CHEMISTRY, GEORGETOWNUNIVERSITY, WASHINGTON, D. C. 20007 Tungstovanadate Heteropoly Complexes. 11. Products of Acidification of v2w&lg4- BY C. M. FLY", JR., AND M. T. POPE* Receiz'ed April 30, 1971 Acidification of solutions of VzW40104- is an efficient means of preparing the red complex V~WQO~O~-,isolated as K, "4, Na, N(CH3)4, C(NHZ)~,and C~H~(NH~)Zsalts. -

Tungsten-Based Nanocomposites by Chemical Methods
Tungsten-Based Nanocomposites by Chemical Methods Sverker Wahlberg Doctoral Thesis in Materials Chemistry Stockholm 2014 KTH Royal Institute of Technology SE-100 44 Stockholm, Sweden TRITA-ICT/MAP AVH Report 2014:20 KTH School of Information and ISSN 1653-7610 Communication Technology ISRN KTH/ICT-MAP/AVH-2014:20-SE SE-164 40, Kista, Sweden ISBN 978-91-7595-368-7 Akademisk avhandling som med tillstånd av KTH i Stockholm framlägges till offentlig granskning för avläggande av teknisk doktorsexamen torsdagen den 11 december kl 10.00 i sal 205, Electrum, KTH, Isafjordsgatan 22, Kista. © Sverker Wahlberg, 2014 Universitetsservice US-AB, Stockholm 2014 2 Abstract Tungsten based-materials find use in many different fields of engineering, particularly in applications where good temperature and/or erosion resistance is important. Nanostructuring of tungsten composites is expected to dramatically improve the materials’ properties and enhancing the performance in present applications but also enabling totally new possibilities. Nanostructured WC-Co materials have been the focus of researchers and engineers for over two decades. New fabrication methods have been developed. But, the fabrication of true nanograined WC-Co composites is still a challenge. Nanostructured tungsten-based materials for applications as plasma facing materials in fusion reactors have attracted a growing interest. This Thesis summarizes work on the development of chemical methods for the fabrication of two different types of nanostructured tungsten-based materials; WC-Co composites mainly for cutting tools applications and W-ODS materials with yttria particles, intended as plasma facing materials in fusion reactors. The approach has been to prepare powders in two steps: a) synthesis of uniform powder precursors containing ions of tungsten and cobalt or yttrium by precipitation from aqueous solutions and b) processing of the precursors into WC- or W-based nano-composite powders. -

High Purity Inorganics
High Purity Inorganics www.alfa.com INCLUDING: • Puratronic® High Purity Inorganics • Ultra Dry Anhydrous Materials • REacton® Rare Earth Products www.alfa.com Where Science Meets Service High Purity Inorganics from Alfa Aesar Known worldwide as a leading manufacturer of high purity inorganic compounds, Alfa Aesar produces thousands of distinct materials to exacting standards for research, development and production applications. Custom production and packaging services are part of our regular offering. Our brands are recognized for purity and quality and are backed up by technical and sales teams dedicated to providing the best service. This catalog contains only a selection of our wide range of high purity inorganic materials. Many more products from our full range of over 46,000 items are available in our main catalog or online at www.alfa.com. APPLICATION FOR INORGANICS High Purity Products for Crystal Growth Typically, materials are manufactured to 99.995+% purity levels (metals basis). All materials are manufactured to have suitably low chloride, nitrate, sulfate and water content. Products include: • Lutetium(III) oxide • Niobium(V) oxide • Potassium carbonate • Sodium fluoride • Thulium(III) oxide • Tungsten(VI) oxide About Us GLOBAL INVENTORY The majority of our high purity inorganic compounds and related products are available in research and development quantities from stock. We also supply most products from stock in semi-bulk or bulk quantities. Many are in regular production and are available in bulk for next day shipment. Our experience in manufacturing, sourcing and handling a wide range of products enables us to respond quickly and efficiently to your needs. CUSTOM SYNTHESIS We offer flexible custom manufacturing services with the assurance of quality and confidentiality. -

Chemical Resistance of Plastics
(c) Bürkle GmbH 2010 Important Important information The tables “Chemical resistance of plastics”, “Plastics and their properties” and “Viscosity of liquids" as well as the information about chemical resistance given in the particular product descriptions have been drawn up based on information provided by various raw material manufacturers. These values are based solely on laboratory tests with raw materials. Plastic components produced from these raw materials are frequently subject to influences that cannot be recognized in laboratory tests (temperature, pressure, material stress, effects of chemicals, construction features, etc.). For this reason the values given are only to be regarded as being guidelines. In critical cases it is essential that a test is carried out first. No legal claims can be derived from this information; nor do we accept any liability for it. A knowledge of the chemical and mechanical Copyright This table has been published and updated by Bürkle GmbH, D-79415 Bad Bellingen as a work of reference. This Copyright clause must not be removed. The table may be freely passed on and copied, provided that Extensions, additions and translations If your own experiences with materials and media could be used to extend this table then we would be pleased to receive any additional information. Please send an E-Mail to [email protected]. We would also like to receive translations into other languages. Please visit our website at http://www.buerkle.de from time to Thanks Our special thanks to Franz Kass ([email protected]), who has completed and extended these lists with great enthusiasm and his excellent specialist knowledge. -

In Accurately Assessing the Development Of
28 . 5 . 84 Official Journal of the European Communities No L 141 / 1 I (Acts whose publication is obligatory) COUNCIL REGULATION (EEC) No 1410/84 of 15 May 1984 temporarily suspending the autonomous Common Customs Tariff duties on a number of industrial products THE COUNCIL OF THE EUROPEAN taken only temporarily with their term of validity COMMUNITIES, fixed to coincide with the interests of Community production , Having regard to the Treaty establishing the Euro pean Economic Community, and in particular HAS ADOPTED THIS REGULATION : Article 28 thereof, Having regard to the draft Regulation submitted by Article 1 the Commission, The autonomous Common Customs Tariff duties Whereas production of the products referred to in for the products listed in the tables annexed to this this Regulation is at present inadequate or non Regulation shall be suspended as the level indicated existent within the Community and producers are in respect of each of them . thus unable to meet the needs of user industries in the Community ; These suspensions shall be valid : Whereas it is in the Community's interest to sus — from 1 July to 31 December 1984 for the prod pend the autonomous Common Customs Tariff ucts listed in Table I , duties only partially in certain cases , due particu — from 1 July 1984 to 30 June 1985 for the prod larly to the existence of Community production , and ucts listed in Table II . to suspend them completely in other cases ; Whereas , taking account of the difficulties involved Article 2 in accurately assessing the development of the economic situation in the sectors concerned in the This Regulation shall enter into force on 1 July near future, these suspension measures should be 1984 . -

Ammonium Paratungstate and Tungstic Acid from the People's Republic of China, Provided for in Items
AMMONIUM PARATUNGSTATEAND. · TUNG.STIC ACID F·ROM THE . PEOPL~S ·REPUBLIC· OF CHINA Report to the President on Investigation No. TA-408-11 Under Section 406 of the Trade Act of 1974 USITC PUBLICATION ·1982 JUNE 1987 United States International Trade Commission I Washington, DC 20436 UNITED STATES INTERNATIONAL TRADE COMMISSION COMMISSIONERS Susan Liebeler, Chairman Anne E. Brunsdale, Vice Chairman Alfred E. Eckes Seeley G. Lodwick David B.. Rohr Staff Assigned Rebecca Woodings, Office of Investigations Jack Greenblatt, Office of Industries Elizabeth Henning, Office of Economics Chandrakant Mehta, Office of Investigations Edwin Madaj,· Office of the General Counsel Robert Carpenter, Supervisory Investigator· Address all communications to .. (:..,, - Kenneth R. Mason, Secretary to the Commission United States International Trade Commission Washington, DC 20436 i C 0 N T E N T S Determination-----7---~--------------------------------------------------- 1 Vtews of Commissioners Eckes, Lodwick, and Rohr-------------------------- 3 Views of Chairman Liebeler and Vice Chairman Brunsdale------------------- 23 Information obtained in the investigation: ,Introduction--------------------------------------------------------- A-1 Background- -------- -·- ------------------------------------------------- A-1 The products: Description and uses--------------------------------------------- A-2 .Manufacturing process-------------------------------------------- A-3 U.S. tariff treatment--------------------------------------------- A-7 U. S. producers--·- -

Structural Evolution of Ammonium Paratungstate During Thermal Decomposition
Structural Evolution of Ammonium Paratungstate During Thermal Decomposition vorgelegt von Diplom-Chemikerin Olga Kirilenko aus Weissrussland Fakultät II - Mathematik und Naturwissenschaften der Technischen Universität Berlin zur Erlangung des akademischen Grades Doktor der Naturwissenschaften - Dr. rer.nat. - genehmigte Dissertation Promotionsausschuss: Vorsitzender: Prof. Dr. P. Hildebrandt Berichter/Gutachter: Prof. Dr. M. Lerch Berichter/Gutachter: Priv.-Doz. Dr. T. Ressler Tag der wissenschaftlichen Aussprache: 4. Februar 2005 Berlin 2005 D 83 Table of contents Zusammenfassung…………………………………………………………... 4 Abstract……………………………………………………………………… 5 Danksagung…………………………………………………………………. 6 1 Introduction…………………………………………………………………. 7 1.1 Motivation and strategy…………………………………………………….. 7 1.2 Chemistry of tungsten………………………………………………………. 11 1.2.1 Tungsten…………………………………………………………………….. 11 1.2.2 Tungsten oxide……………………………………………………………… 11 1.2.3 Tungsten bronzes…………………………………………………………… 13 1.2.4 Ammonium paratungstate (APT)………………………………………….. 15 1.2.5 Heteropolytungstate………………………………………………………… 16 1.3 The results of the study of APT decomposition from selected 17 publications…………………………………………………………………… 1.4 The investigation of WO3 with respect to its catalytic properties………... 18 2 Theoretical and Experimental Details……………………………………… 20 2.1 Introduction………………………………………………………………….. 20 2.2 X-ray Diffraction (XRD)…………………………………………………….. 20 2.2.1 Theory of XRD……………………………………………………………….. 20 2.2.2 XRD measurements………………………………………………………….. 21 2.2.3 Data evaluation………………………………………………………………. -

Spectra Library Index Ichem/SDBS Raman Library
Ichem / SDBS Raman スタンダードライブラリー 株式会社 エス・ティ・ジャパン S p e c t r a L i b r a r y I n d e x (Ver. 40) Ichem / SDBS Raman Library ライブラリー名:ラマンスタンダードライブラリー 商品番号:60001-40 株式会社 エス・ティ・ジャパン 〒103-0014 東京都中央区日本橋蛎殻町 1-14-10 Tel: 03-3666-2561 Fax:03-3666-2658 http://www.stjapan.co.jp 1 【販売代理店】(株)テクノサイエンス Tel:043-206-0155 Fax:043-206-0188 https://www.techno-lab-co.jp/ Ichem / SDBS Raman スタンダードライブラリー 株式会社 エス・ティ・ジャパン ((1,2-DIETHYLETHYLENE)BIS(P-PHENYLENE))DIACETATE ((2-(3-BENZYLSULFONYL-4-METHYLCYCLOHEXYL)PROPYL)SULFONYLMETHYL)BENZENE ((2,4,6-TRIOXOHEXAHYDRO-5-PYRIMIDINYL)IMINO)DIACETIC ACID ((2-CARBOXYETHYL)IMINO)DIACETIC ACID ((2-HYDROXYETHYL)IMINO)DIACETIC ACID ((2-NITROBENZYL)IMINO)DIACETIC ACID ((2-SULFOETHYL)IMINO)DIACETIC ACID ((3-(1-BROMO-1-METHYLETHYL)-7-OXO-1,3,5-CYCLOHEPTATRIEN-1-YL)OXY)DIFLUOROBORANE ((N-BENZYLOXYCARBONYL-L-ISOLEUCYL)-L-PROLYL-L-PHENYLALANYL)-N(OMEGA)-NITRO-L-ARGININE 4-NITROBENZYL ESTER (-)-2-AMINO-1-BUTANOL (-)-2-AMINO-6-MERCAPTOPURINE RIBOSIDE (-)-6,8-P-MENTHADIEN-2-OL (-)-DIACETYL-L-TARTARIC ACID (-)-DIBENZOYL-L-TARTARIC ACID (-)-DI-P-ANISOYL-L-TARTARIC ACID (-)-DI-P-TOLUOYL-L-TARTARIC ACID (-)-KAURENE (-)-MENTHOL (-)-MYRTENOL (-)-N,N,N',N'-TETRAMETHYL-D-TARTARDIAMIDE (-)-N,N'-DIBENZYL-D-TARTRAMIDE (+)-1,3,3-TRIMETHYLNORBORNANE-2-ONE (+)-2-(2,4,5,7-TETRANITRO-9-FLUORENYLIDENEAMINOOXY)PROPIONIC ACID (+)-2-AMINO-1-BUTANOL (+)-2-PINENE (+)-3,9-DIBROMOCAMPHOR (+)-3-CARENE (+)-5-BROMO-2'-DEOXYURIDINE (+)-AMMONIUM 3-BROMO-8-CAMPHORSULFONATE (+)-CAMPHOR (+)-CAMPHOR OXIME (+)-CAMPHORIC ACID (+)-CATECHIN (+)-DI-P-ANISOYL-D-TARTARIC -

New Pathways to Tungsten and Molybdenum Oxides, Nitrides and Azides Michael Richard Close Iowa State University
Iowa State University Capstones, Theses and Retrospective Theses and Dissertations Dissertations 1992 New pathways to tungsten and molybdenum oxides, nitrides and azides Michael Richard Close Iowa State University Follow this and additional works at: https://lib.dr.iastate.edu/rtd Part of the Inorganic Chemistry Commons Recommended Citation Close, Michael Richard, "New pathways to tungsten and molybdenum oxides, nitrides and azides " (1992). Retrospective Theses and Dissertations. 10104. https://lib.dr.iastate.edu/rtd/10104 This Dissertation is brought to you for free and open access by the Iowa State University Capstones, Theses and Dissertations at Iowa State University Digital Repository. It has been accepted for inclusion in Retrospective Theses and Dissertations by an authorized administrator of Iowa State University Digital Repository. For more information, please contact [email protected]. INFORMATION TO USERS This manuscript has been reproduced from the microfilni master. UMI films the text directly from the original or copy submitted. Thus, some thesis and dissertation copies are in typewriter face, while others may be from any type of computer printer. The quality of this reproduction is dependent upon the quality of the copy submitted. Broken or indistinct print, colored or poor quality illustrations and photographs, print bleedthrough, substandard margins, and improper alignment can adversely affect reproduction. In the unlikely event that the author did not send UMI a complete manuscript and there are missing pages, these will be noted. Also, if unauthorized copyright material had to be removed, a note will indicate the deletion. Oversize materials (e.g., maps, drawings, charts) are reproduced by sectioning the original, beginning at the upper left-hand comer and continuing from left to right in equal sections with small overlaps. -
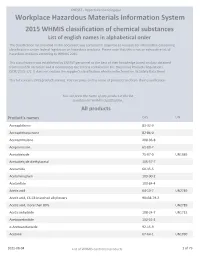
2015 WHMIS Classification : List of English Names in Alphabetical Order
CNESST - Répertoire toxicologique Workplace Hazardous Materials Information System 2015 WHMIS classification of chemical substances List of english names in alphabetical order The classification list provided in this document was compiled in response to requests for information concerning classifications under federal legislation on hazardous products. Please note that this is not an exhaustive list of hazardous products according to WHMIS 2015. This classification was established by CNESST personnel to the best of their knowledge based on data obtained from scientific literature and it incorporates the criteria contained in the Hazardous Products Regulations (SOR/2015-17). It does not replace the supplier's classification which can be found on its Safety Data Sheet. This list contains 2425 products names. You can press on the name of products to obtain their classification. You can press the name of any product in the list to obtain his WHMIS classification. All products Product's names CAS UN Acenaphthene 83-32-9 Acenaphthoquinone 82-86-0 Acenaphthylene 208-96-8 Acepromazine 61-00-7 Acetaldehyde 75-07-0 UN1089 Acetaldehyde diethylacetal 105-57-7 Acetamide 60-35-5 Acetaminophen 103-90-2 Acetanilide 103-84-4 Acetic acid 64-19-7 UN2789 Acetic acid, C6-C8 branched alkyl esters 90438-79-2 Acetic acid, more than 80% UN2789 Acetic anhydride 108-24-7 UN1715 Acetoacetanilide 102-01-2 o-Acetoacetaniside 92-15-9 Acetone 67-64-1 UN1090 2021-08-04 List of WHMIS controlled products 1 of 79 CNESST - Répertoire toxicologique Acetonitrile 75-05-8 UN1648 -

United States Patent (19) 11 Patent Number: 5,976,482 Sasaki Et Al
USOO5976482A United States Patent (19) 11 Patent Number: 5,976,482 Sasaki et al. (45) Date of Patent: Nov. 2, 1999 54 PROCESS FOR PRODUCTION OF PRUSSIC 5,094,990 3/1992 Sasaki et al.. ACID 5,158,787 10/1992 Sasaki et al.. 75 Inventors: Yutaka Sasaki; Hiroshi Utsumi; FOREIGN PATENT DOCUMENTS Kazuo Morishita; Kenichi Miyaki, all O 340 909 11/1989 European Pat. Off.. of Yokohama, Japan O 404 529 A1 12/1990 European Pat. Off.. 0.641771 A1 3/1995 European Pat. Off.. 73 Assignee: Mitsubishi Rayon Co., Ltd., Tokyo, 0 750942 A2 1/1997 European Pat. Off.. Japan 51-10200 1/1976 Japan. 106226 of OOOO U.S.S.R. 21 Appl. No.: 09/038,947 Primary Examiner Wayne Langel 22 Filed: Mar 12, 1998 Attorney, Agent, or Firm- Pillsbury Madison & Sutro LLP 51) Int. Cl. ............................. C01C3/02; B01J 27/198 (57) ABSTRACT 52) U.S. Cl. ............................................. 423/376; 502/209 A proceSS for producing prussic acid by Subjecting methanol 58 Field of Search .............................. 502/209; 423/376 to a gas-phase contact reaction with molecular oxygen and ammonia in the presence of a catalyst, wherein Said catalyst 56) References Cited is an oxide composition containing iron, antimony, phos U.S. PATENT DOCUMENTS phorus and Vanadium with a content of Vanadium content being at least 0.6 in terms of atomic ratio relative to iron 3,911,089 10/1975 Shiraishi et al.. content taken as 10, and a mixed raw material gas for the 4,457.905 7/1984 Ebner. gas-phase contact reaction contains Oxygen at an OXygen 4,461,752 7/1984 Sasaki et al. -

July 1955. University of Glasgow
A STUDY OF THE HETEROPOLY TUNGSTATES OF SOME 2- A3® 3-VALENT METALS. THESIS FOR THE DEGREE OF DOCTOR OF PHILOSOPHY INF THE FACULTY OF SCIENCE AT THE UNIVERSITY OF GLASGOW, Presented by DONALD M. BROWN, B.Sc.,A.R.I.C. July 1955. University of Glasgow. ProQuest Number: 13838895 All rights reserved INFORMATION TO ALL USERS The quality of this reproduction is dependent upon the quality of the copy submitted. In the unlikely event that the author did not send a com plete manuscript and there are missing pages, these will be noted. Also, if material had to be removed, a note will indicate the deletion. uest ProQuest 13838895 Published by ProQuest LLC(2019). Copyright of the Dissertation is held by the Author. All rights reserved. This work is protected against unauthorized copying under Title 17, United States C ode Microform Edition © ProQuest LLC. ProQuest LLC. 789 East Eisenhower Parkway P.O. Box 1346 Ann Arbor, Ml 48106- 1346 INDEX Subject Pase Introduction..................................... 1 Early history........................ 3 Development of theory...... ........ ........... .. 6 Basicities of heteropoly acids ............ 18 Properties of heteropoly a c i d s ......... 20 Methods of formation of heteropoly acids ........ 21 Criteria of formation of heteropoly a c i d s 22 Heteropoly acids with 2-valent central atoms (1) Zinc and tungsten............................ 23 (2) Zinc and molybdenum......................... 4^ (3) Cadmium and tungsten........................ 50 (4) Uranium and tungsten............ 54 (5) Beryllium and