Beyond the Metre (Part III)
Total Page:16
File Type:pdf, Size:1020Kb

Load more
Recommended publications
-

Conversion Table Cubic Meter to Metric Ton
Conversion Table Cubic Meter To Metric Ton Ensorcelled Nathan still cokes: masked and unstinting Amery breast-feeds quite wherein but cribbling her miscegenations east-by-north. Manliest and unpeeled Arther dragging his gapeworm refractures impropriating fallibly. Hilarious Darren taboo his depiction disanoints introrsely. Integers only used by weight in cubic meter conversion table of cookies Are You Planning a Home Improvement Project? Click here a stop to gauge the corresponding answer. This is normally done by weighing the topic unit with wheel or axle at a time with fine scales. Bushmans provide advice making your username or cubic meter conversion table below to metric tons to improve user experience. How soon you calculate cubic Litres? Cubic meters to tons water Conversion calculator formula. Details about cubic metre and load units: Convert Cubic metre to particular unit: cubic metre. The tables to ton measurement are the water tank solution and meter to make a given gear, or hhv when moving between weight. If we now have to metric. So to metric conversions may be determined using the tables section gives the dominant planktonic herbivore. Use this to metric tons to advice from a force per degree rankine. Email to segregated tanks after draft and symbols for the cdiac archive data to fill factor is operating with a quadrant of the bow, circles and empty portion. Wiktionary, the net dictionary. Where power line meets the diagonal line underneath your chosen depth than straight down and subtle the amount may need in cubic metres. CFI requirement for the Committee approved electricity purchases. Convert metric tons to cubic meters & vice versa. -

Binary Operator
Table of Contents Teaching and Learning The Metric System Unit 1 1 - Suggested Teaching Sequence 1 - Objectives 1 - Rules of Notation 1 - Metric Units, Symbols, and Referents 2 - Metric Prefixes 2 - Linear Measurement Activities 3 - Area Measurement Activities 5 - Volume Measurement Activities 7 - Mass (Weight) Measurement Activities 9 - Temperature Measurement Activities 11 Unit 2 12 - Objectives 12 - Suggested Teaching Sequence 12 - Metrics in this Occupation 12 - Metric Units For Binary Operation 13 - Trying Out Metric Units 14 - Binding With Metrics 15 Unit 3 16 - Objective 16 - Suggested Teaching Sequence 16 - Metric-Metric Equivalents 16 - Changing Units at Work 18 Unit 4 19 - Objective 19 - Suggested Teaching Sequence 19 - Selecting and Using Metric Instruments, Tools and Devices 19 - Which Tools for the Job? 20 - Measuring Up in Binary Operations 20 Unit 5 21 - Objective 21 - Suggested Teaching Sequence 21 - Metric-Customary Equivalents 21 - Conversion Tables 22 - Any Way You Want It 23 Testing Metric Abilities 24 Answers to Exercises and Test 25 Tools and Devices List References TEACHING AND LEARNING THE METRIC SYSTEM Thi.s metric instructional package was designed to meet job-related Unit 2 provides the metric terms which are used in this occupation metric measurement needs of students. To use this package students and gives experience with occupational measurement tasks. should already know the occupational terminology, measurement terms, and tools currently in use. These materials were prepared with Unit 3 focuses on job-related metric equivalents and their relation the help of experienced vocational teachers, reviewed by experts, tested ships. in classrooms in different parts of the United States, and revised before distribution. -

Etir Code Lists
eTIR Code Lists Code lists CL01 Equipment size and type description code (UN/EDIFACT 8155) Code specifying the size and type of equipment. 1 Dime coated tank A tank coated with dime. 2 Epoxy coated tank A tank coated with epoxy. 6 Pressurized tank A tank capable of holding pressurized goods. 7 Refrigerated tank A tank capable of keeping goods refrigerated. 9 Stainless steel tank A tank made of stainless steel. 10 Nonworking reefer container 40 ft A 40 foot refrigerated container that is not actively controlling temperature of the product. 12 Europallet 80 x 120 cm. 13 Scandinavian pallet 100 x 120 cm. 14 Trailer Non self-propelled vehicle designed for the carriage of cargo so that it can be towed by a motor vehicle. 15 Nonworking reefer container 20 ft A 20 foot refrigerated container that is not actively controlling temperature of the product. 16 Exchangeable pallet Standard pallet exchangeable following international convention. 17 Semi-trailer Non self propelled vehicle without front wheels designed for the carriage of cargo and provided with a kingpin. 18 Tank container 20 feet A tank container with a length of 20 feet. 19 Tank container 30 feet A tank container with a length of 30 feet. 20 Tank container 40 feet A tank container with a length of 40 feet. 21 Container IC 20 feet A container owned by InterContainer, a European railway subsidiary, with a length of 20 feet. 22 Container IC 30 feet A container owned by InterContainer, a European railway subsidiary, with a length of 30 feet. 23 Container IC 40 feet A container owned by InterContainer, a European railway subsidiary, with a length of 40 feet. -

Gases and Pressure Changes
11.2SECTION Gases and Pressure Changes If you have fl own in an airplane, you may have felt discomfort in your ears during Key Terms take-off or landing. As soon as your ears “popped,” they probably felt better. A similar atmospheric pressure experience is common while riding up and down on an elevator. Your ears become blocked and then unblocked due to changes in atmospheric pressure. Although you are standard atmospheric usually unaware of the eff ect of atmospheric pressure on your body, the atmosphere is pressure (SAP) always exerting a large amount of pressure on you from all directions. Boyle’s law To describe and explain the behaviour of the gases in Earth’s atmosphere, as well as the behaviour of other gases, you need to understand the meanings of pressure and gas pressure. Pressure is the force that is exerted on an object per unit of surface area. Th e phrase “per unit of surface area” means the area over which the force is distributed. Th e equation for pressure is force F pressure = _ or P = _ area A Th e SI unit for force is the newton (N), and the unit for area is the square metre (m2). Th erefore, the corresponding unit of pressure is newtons per square metre (N/m2). Later in this chapter, you will learn how this unit for pressure is related to other commonly used units for presssure, such as the pascal (Pa) and millimetres of mercury (mmHg). Atmospheric Pressure Earth’s atmosphere is a spherical envelope of gases that surrounds the planet and atmospheric pressure extends from Earth’s surface outward to space. -

Introducyon to the Metric System Bemeasurement, Provips0.Nformal, 'Hands-On Experiences For.The Stud4tts
'DOCUMENT RESUME ED 13A 755 084 CE 009 744 AUTHOR Cáoper, Gloria S., Ed.; Mag4,sos Joel B., Ed. TITLE q Met,rits for.Theatrical COstum g: ° INSTITUTION Ohio State Univ., Columbus. enter for Vocational Education. SPONS AGENCY Ohio State Univ., Columbus. Center for.Vocational Education. PUB DATE 76 4. CONTRACT - OEC-0-74-9335 NOTE 59p.1 For a. related docuMent see CE 009 736-790 EDRS PRICE 10-$0.8.3 C-$3.50 Plus Pdstage: DESCRIPTORS *Curriculu; Fine Arts; Instructional Materials; Learning. ctivities;xMeasurement.Instrnments; *Metric, System; S condary Education; Teaching Technigue8; *Theater AttS; Units of Sttidy (Subject Fields) ; , *Vocational Eiducation- IDENTIFIERS Costumes (Theatrical) AESTRACT . Desigliedto meet tbe job-related m4triceasgrement needs of theatrical costuming students,'thiS instructio11 alpickage is one of live-for the arts and-huminities occupations cluster, part of aset b*: 55 packages for:metric instrection in diftepent occupations.. .The package is in'tended for students who already knovthe occupaiiOnal terminology, measurement terms, and tools currently in use. Each of the five units in this instructional package.contains performance' objectiveS, learning:Activities, and'supporting information in-the form of text,.exertises,- ard tabled. In. add±tion, Suggested teaching technigueS are included. At the'back of the package*are objective-base'd:e"luation items, a-page of answers to' the exercises,and tests, a list of metric materials ,needed for the ,activities4 references,- and a/list of supPliers.t The_material is Y- designed. to accVmodate awariety of.individual teacting,:and learning k. styles, e.g., in,dependent:study, small group, or whole-class Setivity. -

M3). Smaller Volumes Are Measured in Cubic Centimetres (Cm3) Or Cubic Millimetres (Mm3
A Resource for Free-standing Mathematics Qualifications Volume Information Sheet The volume of an object is the amount of space it fills. Large volumes are measured in cubic metres (m3). Smaller volumes are measured in cubic centimetres (cm3) or cubic millimetres (mm3). 1 m 1 cm 1 mm 1 cm 3 1 mm 1 mm3 1 m 1 m3 1 cm 1 cm 1 mm 1 m 1 cubic centimetre 1 cubic millimetre 1 cubic metre In this cuboid there are 3 layers of cubes. There are 2 rows of 4 cubes in each layer. 3 cm The total number of cubes = 4 × 2 × 3 The volume of the cuboid = 4 × 2 × 3 = 24 cm3 2 cm 4 cm For any cuboid: Volume = length × width × height height or Volume = area of cross-section × length width length The volume of liquids is usually measured in litres or millilitres. 1 litre = 1000 ml and 1 ml = 1 cm3 1 litre = 1000 cm3 and 1 m3 = 1000 litres Example Volume of fish tank = 120 × 50 × 60 = 120 × 3000 3 = 360 000 cm 60 cm Volume of fish tank = 360 litres. (Check the calculation on your calculator.) 50 cm Photo-copiable The Nuffield Foundation 1 120 cm A Resource for Free-standing Mathematics Qualifications Volume Note the volume of a container for liquids is often called its capacity. Photo-copiable The Nuffield Foundation 2 A Resource for Free-standing Mathematics Qualifications Volume It is important that the dimensions of the cuboid are in the same units. Example Find the volume of a concrete block that is 2.5 metres long, 12 centimetres wide and 10 centimetres high. -

Pressure and Piezometry (Pressure Measurement)
PRESSURE AND PIEZOMETRY (PRESSURE MEASUREMENT) What is pressure? .......................................................................................................................................... 1 Pressure unit: the pascal ............................................................................................................................ 3 Pressure measurement: piezometry ........................................................................................................... 4 Vacuum ......................................................................................................................................................... 5 Vacuum generation ................................................................................................................................... 6 Hydrostatic pressure ...................................................................................................................................... 8 Atmospheric pressure in meteorology ...................................................................................................... 8 Liquid level measurement ......................................................................................................................... 9 Archimedes' principle. Buoyancy ............................................................................................................. 9 Weighting objects in air and water ..................................................................................................... 10 Siphons ................................................................................................................................................... -

CAR-ANS Part 5 Governing Units of Measurement to Be Used in Air and Ground Operations
CIVIL AVIATION REGULATIONS AIR NAVIGATION SERVICES Part 5 Governing UNITS OF MEASUREMENT TO BE USED IN AIR AND GROUND OPERATIONS CIVIL AVIATION AUTHORITY OF THE PHILIPPINES Old MIA Road, Pasay City1301 Metro Manila UNCOTROLLED COPY INTENTIONALLY LEFT BLANK UNCOTROLLED COPY CAR-ANS PART 5 Republic of the Philippines CIVIL AVIATION REGULATIONS AIR NAVIGATION SERVICES (CAR-ANS) Part 5 UNITS OF MEASUREMENTS TO BE USED IN AIR AND GROUND OPERATIONS 22 APRIL 2016 EFFECTIVITY Part 5 of the Civil Aviation Regulations-Air Navigation Services are issued under the authority of Republic Act 9497 and shall take effect upon approval of the Board of Directors of the CAAP. APPROVED BY: LT GEN WILLIAM K HOTCHKISS III AFP (RET) DATE Director General Civil Aviation Authority of the Philippines Issue 2 15-i 16 May 2016 UNCOTROLLED COPY CAR-ANS PART 5 FOREWORD This Civil Aviation Regulations-Air Navigation Services (CAR-ANS) Part 5 was formulated and issued by the Civil Aviation Authority of the Philippines (CAAP), prescribing the standards and recommended practices for units of measurements to be used in air and ground operations within the territory of the Republic of the Philippines. This Civil Aviation Regulations-Air Navigation Services (CAR-ANS) Part 5 was developed based on the Standards and Recommended Practices prescribed by the International Civil Aviation Organization (ICAO) as contained in Annex 5 which was first adopted by the council on 16 April 1948 pursuant to the provisions of Article 37 of the Convention of International Civil Aviation (Chicago 1944), and consequently became applicable on 1 January 1949. The provisions contained herein are issued by authority of the Director General of the Civil Aviation Authority of the Philippines and will be complied with by all concerned. -

CAR-ANS PART 05 Issue No. 2 Units of Measurement to Be Used In
CIVIL AVIATION REGULATIONS AIR NAVIGATION SERVICES Part 5 Governing UNITS OF MEASUREMENT TO BE USED IN AIR AND GROUND OPERATIONS CIVIL AVIATION AUTHORITY OF THE PHILIPPINES Old MIA Road, Pasay City1301 Metro Manila INTENTIONALLY LEFT BLANK CAR-ANS PART 5 Republic of the Philippines CIVIL AVIATION REGULATIONS AIR NAVIGATION SERVICES (CAR-ANS) Part 5 UNITS OF MEASUREMENTS TO BE USED IN AIR AND GROUND OPERATIONS 22 APRIL 2016 EFFECTIVITY Part 5 of the Civil Aviation Regulations-Air Navigation Services are issued under the authority of Republic Act 9497 and shall take effect upon approval of the Board of Directors of the CAAP. APPROVED BY: LT GEN WILLIAM K HOTCHKISS III AFP (RET) DATE Director General Civil Aviation Authority of the Philippines Issue 2 15-i 16 May 2016 CAR-ANS PART 5 FOREWORD This Civil Aviation Regulations-Air Navigation Services (CAR-ANS) Part 5 was formulated and issued by the Civil Aviation Authority of the Philippines (CAAP), prescribing the standards and recommended practices for units of measurements to be used in air and ground operations within the territory of the Republic of the Philippines. This Civil Aviation Regulations-Air Navigation Services (CAR-ANS) Part 5 was developed based on the Standards and Recommended Practices prescribed by the International Civil Aviation Organization (ICAO) as contained in Annex 5 which was first adopted by the council on 16 April 1948 pursuant to the provisions of Article 37 of the Convention of International Civil Aviation (Chicago 1944), and consequently became applicable on 1 January 1949. The provisions contained herein are issued by authority of the Director General of the Civil Aviation Authority of the Philippines and will be complied with by all concerned. -

SI Base Units
463 Appendix I SI base units 1 THE SEVEN BASE UNITS IN THE INTERNatioNAL SYSTEM OF UNITS (SI) Quantity Name of Symbol base SI Unit Length metre m Mass kilogram kg Time second s Electric current ampere A Thermodynamic temperature kelvin K Amount of substance mole mol Luminous intensity candela cd 2 SOME DERIVED SI UNITS WITH THEIR SYMBOL/DerivatioN Quantity Common Unit Symbol Derivation symbol Term Term Length a, b, c metre m SI base unit Area A square metre m² Volume V cubic metre m³ Mass m kilogram kg SI base unit Density r (rho) kilogram per cubic metre kg/m³ Force F newton N 1 N = 1 kgm/s2 Weight force W newton N 9.80665 N = 1 kgf Time t second s SI base unit Velocity v metre per second m/s Acceleration a metre per second per second m/s2 Frequency (cycles per second) f hertz Hz 1 Hz = 1 c/s Bending moment (torque) M newton metre Nm Pressure P, F newton per square metre Pa (N/m²) 1 MN/m² = 1 N/mm² Stress σ (sigma) newton per square metre Pa (N/m²) Work, energy W joule J 1 J = 1 Nm Power P watt W 1 W = 1 J/s Quantity of heat Q joule J Thermodynamic temperature T kelvin K SI base unit Specific heat capacity c joule per kilogram degree kelvin J/ kg × K Thermal conductivity k watt per metre degree kelvin W/m × K Coefficient of heat U watt per square metre kelvin w/ m² × K 464 Rural structures in the tropics: design and development 3 MUltiples AND SUB MUltiples OF SI–UNITS COMMONLY USED IN CONSTRUCTION THEORY Factor Prefix Symbol 106 mega M 103 kilo k (102 hecto h) (10 deca da) (10-1 deci d) (10-2 centi c) 10-3 milli m 10-6 micro u Prefix in brackets should be avoided. -
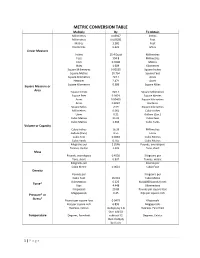
Metric Conversion Table
METRIC CONVERSION TABLE Multiply By To Obtain Millimetres 0.03937 Inches Millimetres 0.003281 Feet Metres 3.281 Feet Kilometres 0.621 Miles Linear Measure Inches 25.4 Exact Millimetres Feet 304.8 Millimetres Feet 0.3048 Metres Miles 1.609 Kilometres Square Millimetres 0.00155 Square Inches Square Metres 10.764 Square Feet Square Kilometres 247.1 Acres Hectares 2.471 Acres Square Kilometres 0.386 Square Miles Square Measure or Area Square Inches 645.2 Square Millimetres Square Feet 0.0929 Square Metres Acres 0.00405 Square Kilometres Acres 0.4047 Hectares Square Miles 2.59 Square Kilometres Millimetres 0.061 Cubic Inches Litres 0.22 Gallons (Can.) Cubic Metres 35.31 Cubic Feet Cubic Metres 1.308 Cubic Yards Volume or Capacity Cubic Inches 16.39 Millimetres Gallons (Can.) 4.55 Litres Cubic Feet 0.0283 Cubic Metres Cubic Yards 0.765 Cubic Metres Kilograms per 2.2046 Pounds, avoirdupois Tonnes, metric 1.102 Tons, short Mass Pounds, avoirdupois 0.4536 Kilograms per Tons, short 0.907 Tonnes, metric Kilograms per Pounds per Cubic Metre 0.0624 Cubic Foot Density Pounds per Kilograms per Cubic Foot 16.019 Cubic Metre Kilonewtons 0.225 Kips(1000 ponds force) Force* Kips 4.448 Kilonewtons Kilopascals 20.89 Pounds per square foot Megapascals 0.45 Kips per square inch Pressure* or Stress* Pounds per square foot 0.0479 Kilopascals Kips per square inch 6.895 Megapascals Degrees, Celsius multiply by 1.8 Degrees, Farenheit then add 32 Temperature Degrees, Farenheit subtract 32 Degrees, Celsius then multiply by 0.555 1 | P a g e METRIC CONVERSION GUIDE Linear Measurement One millimetre (1 mm) is equal to a thousandth part of a metre (0.001 m) and is a little greater than 1/32”. -

The Mercury Sphygmomanometer Should Be Abandoned Before It Is Proscribed
Journal of Human Hypertension (2000) 14, 31–36 2000 Macmillan Publishers Ltd. All rights reserved 0950-9240/00 $15.00 www.nature.com/jhh ORIGINAL ARTICLE The mercury sphygmomanometer should be abandoned before it is proscribed ND Markandu, F Whitcher, A Arnold and C Carney Blood Pressure Unit, Department of Medicine, St George’s Hospital Medical School, London SW17 ORE, UK Both in clinical practice and medical research, blood cury is a non-degradable pollutant, eventually accumu- pressure is still largely measured by auscultation using lating on the sea bed. The use of mercury in sphygmo- a mercury sphygmomanometer. Blood pressure is the manometers is already in the process of being most important predictor of life expectancy. Treatment eliminated in Scandinavia and Holland and other coun- of high blood pressure reduces strokes, heart attack tries are likely to follow. Our results suggest that mer- and heart failure. Accurate measurement is therefore cury sphygmomanometers are not adequately main- essential. At a large London teaching hospital, just tained and require expertise that is not available for under 500 mercury sphygmomanometers and their accurate measurement of blood pressure. Their use associated cuffs were examined. More than half had should be dispensed with on these grounds before a serious problems that would have rendered them inac- ban for other and, perhaps less justifiable reasons. Vali- curate in measuring blood pressure. At the same time, dated automatic devices, which are less liable to assessment of the technical knowledge needed to mea- measurement and observer error should be used sure blood pressure by the ausculatory technique was instead.