SLIDE—Novel Approach to Apocrine Sweat Sampling for Lipid Profiling in Healthy Individuals
Total Page:16
File Type:pdf, Size:1020Kb

Load more
Recommended publications
-

Skates and Rays Diversity, Exploration and Conservation – Case-Study of the Thornback Ray, Raja Clavata
UNIVERSIDADE DE LISBOA FACULDADE DE CIÊNCIAS DEPARTAMENTO DE BIOLOGIA ANIMAL SKATES AND RAYS DIVERSITY, EXPLORATION AND CONSERVATION – CASE-STUDY OF THE THORNBACK RAY, RAJA CLAVATA Bárbara Marques Serra Pereira Doutoramento em Ciências do Mar 2010 UNIVERSIDADE DE LISBOA FACULDADE DE CIÊNCIAS DEPARTAMENTO DE BIOLOGIA ANIMAL SKATES AND RAYS DIVERSITY, EXPLORATION AND CONSERVATION – CASE-STUDY OF THE THORNBACK RAY, RAJA CLAVATA Bárbara Marques Serra Pereira Tese orientada por Professor Auxiliar com Agregação Leonel Serrano Gordo e Investigadora Auxiliar Ivone Figueiredo Doutoramento em Ciências do Mar 2010 The research reported in this thesis was carried out at the Instituto de Investigação das Pescas e do Mar (IPIMAR - INRB), Unidade de Recursos Marinhos e Sustentabilidade. This research was funded by Fundação para a Ciência e a Tecnologia (FCT) through a PhD grant (SFRH/BD/23777/2005) and the research project EU Data Collection/DCR (PNAB). Skates and rays diversity, exploration and conservation | Table of Contents Table of Contents List of Figures ............................................................................................................................. i List of Tables ............................................................................................................................. v List of Abbreviations ............................................................................................................. viii Agradecimentos ........................................................................................................................ -

Vocabulario De Morfoloxía, Anatomía E Citoloxía Veterinaria
Vocabulario de Morfoloxía, anatomía e citoloxía veterinaria (galego-español-inglés) Servizo de Normalización Lingüística Universidade de Santiago de Compostela COLECCIÓN VOCABULARIOS TEMÁTICOS N.º 4 SERVIZO DE NORMALIZACIÓN LINGÜÍSTICA Vocabulario de Morfoloxía, anatomía e citoloxía veterinaria (galego-español-inglés) 2008 UNIVERSIDADE DE SANTIAGO DE COMPOSTELA VOCABULARIO de morfoloxía, anatomía e citoloxía veterinaria : (galego-español- inglés) / coordinador Xusto A. Rodríguez Río, Servizo de Normalización Lingüística ; autores Matilde Lombardero Fernández ... [et al.]. – Santiago de Compostela : Universidade de Santiago de Compostela, Servizo de Publicacións e Intercambio Científico, 2008. – 369 p. ; 21 cm. – (Vocabularios temáticos ; 4). - D.L. C 2458-2008. – ISBN 978-84-9887-018-3 1.Medicina �������������������������������������������������������������������������veterinaria-Diccionarios�������������������������������������������������. 2.Galego (Lingua)-Glosarios, vocabularios, etc. políglotas. I.Lombardero Fernández, Matilde. II.Rodríguez Rio, Xusto A. coord. III. Universidade de Santiago de Compostela. Servizo de Normalización Lingüística, coord. IV.Universidade de Santiago de Compostela. Servizo de Publicacións e Intercambio Científico, ed. V.Serie. 591.4(038)=699=60=20 Coordinador Xusto A. Rodríguez Río (Área de Terminoloxía. Servizo de Normalización Lingüística. Universidade de Santiago de Compostela) Autoras/res Matilde Lombardero Fernández (doutora en Veterinaria e profesora do Departamento de Anatomía e Produción Animal. -

Embryology and Development of Salivary Gland 1
European Journal of Molecular & Clinical Medicine ISSN 2515-8260 Volume 07, Issue 10, 2020 Embryology and development of salivary gland 1. Dr. Sangeetha Priya.P,Dr.N.Anitha, Dr.E.Rajesh, Dr.K.M.K.Masthan Professor, Department of Oral Pathology and Microbiology Sree Balaji Dental College and Hospital Bharath Institute of Higher Education and Research ABSTRACT: Saliva is the mixed glandular secretion which constantly bathes the teeth and the oral mucosa. It is constituted by the secretions of the three paired major salivary glands; the parotid, submandibular and sublingual . Salivary glands are complex in nature. They could be either tubulo acinar, merocrine or exocrine glands secreting mainly saliva. Salivary gland is one of the main soft tissue structures in the maxillofacial area. This review article illustrates the processes that lead to the development,embryology of the salivary glands and how this relates to the adult anatomy. KEY WORDS: salivary gland embryology, salivary gland development, salivary gland anatomy INTRODUCTION: A gland consists of specialized type of cells, wherein they produce products which are used elsewhere in the body. Salivary glands are complex, tubulo acinar, exocrine or merocrine glands secreting mainly saliva. Saliva is the product of the major and minor salivary gland dispersed throughout the oral cavity. It is a complex mixture of organic, inorganic components and water, carrying out several functions. There are three pairs of major salivary glands namely parotid, sub mandibular and sublingual glands in addition to numerous minor salivary glands in the oral cavity1. Together these glands produce saliva, which contains digestive enzymes, antibodies, growth factors, and coating substances essential for eating, speaking, tasting, and oral hygiene2,3. -

Epithelium 2 : Glandular Epithelium Histology Laboratory -‐ Year 1, Fall Term Dr
Epithelium 2 : Glandular Epithelium Histology Laboratory -‐ Year 1, Fall Term Dr. Heather Yule ([email protected]) October 21, 2014 Slides for study: 75 (Salivary Gland), 355 (Pancreas Tail), 48 (Atrophic Mammary Gland), 49 (Active Mammary Gland) and 50 (Resting Mammary Gland) Electron micrographs for : study EM: Serous acinus in parotid gland EM: Mucous acinus in mixed salivary gland EM: Pancreatic acinar cell Main Objective: Understand key histological features of glandular epithelium and relate structure to function. Specific Objectives: 1. Describe key histological differences between endocrine and exocrine glands including their development. 2. Compare three modes of secretion in glands; holocrine, apocrine and merocrine. 3. Explain the functional significance of polarization of glandular epithelial cells. 4. Define the terms parenchyma, stroma, mucous acinus, serous acinus and serous a demilune and be able to them identify in glandular tissue. 5. Distinguish exocrine and endocrine pancreas. 6. Compare the histology of resting, lactating and postmenopausal mammary glands. Keywords: endocrine gland, exocrine gland, holocrine, apocrine, merocrine, polarity, parenchyma, stroma, acinus, myoepithelial cell, mucous gland, serous gland, mixed or seromucous gland, serous demilune, exocrine pancreas, endocrine pancreas (pancreatic islets), resting mammary gland, lactating mammary gland, postmenopausal mammary gland “This copy is made solely for your personal use for research, private study, education, parody, satire, criticism, or review -

An Analysis of Benign Human Prostate Offers Insights Into the Mechanism
www.nature.com/scientificreports OPEN An analysis of benign human prostate ofers insights into the mechanism of apocrine secretion Received: 12 March 2018 Accepted: 22 February 2019 and the origin of prostasomes Published: xx xx xxxx Nigel J. Fullwood 1, Alan J. Lawlor2, Pierre L. Martin-Hirsch3, Shyam S. Matanhelia3 & Francis L. Martin 4 The structure and function of normal human prostate is still not fully understood. Herein, we concentrate on the diferent cell types present in normal prostate, describing some previously unreported types and provide evidence that prostasomes are primarily produced by apocrine secretion. Patients (n = 10) undergoing TURP were prospectively consented based on their having a low risk of harbouring CaP. Scanning electron microscopy and transmission electron microscopy was used to characterise cell types and modes of secretion. Zinc levels were determined using Inductively Coupled Plasma Mass Spectrometry. Although merocrine secretory cells were noted, the majority of secretory cells appear to be apocrine; for the frst time, we clearly show high-resolution images of the stages of aposome secretion in human prostate. We also report a previously undescribed type of epithelial cell and the frst ultrastructural image of wrapping cells in human prostate stroma. The zinc levels in the tissues examined were uniformly high and X-ray microanalysis detected zinc in merocrine cells but not in prostasomes. We conclude that a signifcant proportion of prostasomes, possibly the majority, are generated via apocrine secretion. This fnding provides an explanation as to why so many large proteins, without a signal peptide sequence, are present in the prostatic fuid. Tere are many complications associated with the prostate from middle age onwards, including benign prostatic hyperplasia (BPH) and prostate cancer (PCa). -
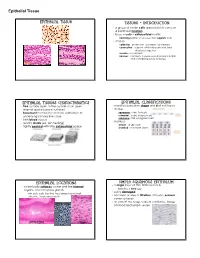
Epithelial Tissue
Epithelial Tissue Epithelial Tissue Tissues - Introduction · a group of similar cells specialized to carry on a particular function · tissue = cells + extracellular matrix nonliving portion of a tissue that supports cells · 4 types epithelial - protection, secretion, absorption connective - support soft body parts and bind structures together muscle - movement nervous - conducts impulses used to help control and coordinate body activities Epithelial Tissues Characteristics Epithelial Classifications · free surface open to the outside or an open · classified based on shape and # of cell layers internal space (apical surface) · shape · basement membrane anchors epithelium to squamous - thin, flat cells underlying connective tissue cuboidal - cube-shaped cells columnar - tall, elongated cells · lack blood vessels · number · readily divide (ex. skin healing) simple - single layer · tightly packed with little extracellular space stratified - 2 or more layers Epithelial Locations Simple Squamous Epithelium · a single layer of thin, flattened cells · cover body surfaces, cover and line internal organs, and compose glands looks like a fried egg · easily damaged skin cells, cells that line the stomach and small intestine, inside your mouth · common at sites of filtration, diffusion, osmosis; cover surfaces · air sacs of the lungs, walls of capillaries, linings cheek cells of blood and lymph vessels intestines skin Epithelial Tissue Simple Cuboidal Epithelium Simple Columnar Epithelium · single layer of cube-shaped cells · single layer of cells -

Nomina Histologica Veterinaria, First Edition
NOMINA HISTOLOGICA VETERINARIA Submitted by the International Committee on Veterinary Histological Nomenclature (ICVHN) to the World Association of Veterinary Anatomists Published on the website of the World Association of Veterinary Anatomists www.wava-amav.org 2017 CONTENTS Introduction i Principles of term construction in N.H.V. iii Cytologia – Cytology 1 Textus epithelialis – Epithelial tissue 10 Textus connectivus – Connective tissue 13 Sanguis et Lympha – Blood and Lymph 17 Textus muscularis – Muscle tissue 19 Textus nervosus – Nerve tissue 20 Splanchnologia – Viscera 23 Systema digestorium – Digestive system 24 Systema respiratorium – Respiratory system 32 Systema urinarium – Urinary system 35 Organa genitalia masculina – Male genital system 38 Organa genitalia feminina – Female genital system 42 Systema endocrinum – Endocrine system 45 Systema cardiovasculare et lymphaticum [Angiologia] – Cardiovascular and lymphatic system 47 Systema nervosum – Nervous system 52 Receptores sensorii et Organa sensuum – Sensory receptors and Sense organs 58 Integumentum – Integument 64 INTRODUCTION The preparations leading to the publication of the present first edition of the Nomina Histologica Veterinaria has a long history spanning more than 50 years. Under the auspices of the World Association of Veterinary Anatomists (W.A.V.A.), the International Committee on Veterinary Anatomical Nomenclature (I.C.V.A.N.) appointed in Giessen, 1965, a Subcommittee on Histology and Embryology which started a working relation with the Subcommittee on Histology of the former International Anatomical Nomenclature Committee. In Mexico City, 1971, this Subcommittee presented a document entitled Nomina Histologica Veterinaria: A Working Draft as a basis for the continued work of the newly-appointed Subcommittee on Histological Nomenclature. This resulted in the editing of the Nomina Histologica Veterinaria: A Working Draft II (Toulouse, 1974), followed by preparations for publication of a Nomina Histologica Veterinaria. -

A Focus on Breast Cancer
HHS Public Access Author manuscript Author ManuscriptAuthor Manuscript Author Pharmacol Manuscript Author Ther. Author Manuscript Author manuscript; available in PMC 2017 May 01. Published in final edited form as: Pharmacol Ther. 2016 May ; 161: 79–96. doi:10.1016/j.pharmthera.2016.03.003. Emerging therapeutic targets in metastatic progression: a focus on breast cancer Zhuo Li and Yibin Kang* Department of Molecular Biology, Princeton University, Princeton, NJ, 08544, United States Abstract Metastasis is the underlying cause of death for the majority of breast cancer patients. Despite significant advances in recent years in basic research and clinical development, therapies that specifically target metastatic breast cancer remain inadequate, and represents the single greatest obstacle to reducing mortality of late-stage breast cancer. Recent efforts have leveraged genomic analysis of breast cancer and molecular dissection of tumor-stromal cross-talk to uncover a number of promising candidates for targeted treatment of metastatic breast cancer. Rational combinations of therapeutic agents targeting tumor-intrinsic properties and microenvironmental components provide a promising strategy to develop precision treatments with higher specificity and less toxicity. In this review, we discuss the emerging therapeutic targets in breast cancer metastasis, from tumor-intrinsic pathways to those that involve the host tissue components, including the immune system. Keywords Breast cancer; Metastasis; Targeted therapy; Tumor microenvironment; Immunotherapy 1. Introduction The overall 5-year survival rate for breast cancer currently stands at 90% — a dramatic improvement over the 63% survival rate in the early 1960s. When stratified by stage, the 5- year survival rates have increased to 99% for localized disease and 85% for regional advanced disease, a trend that can be attributed to early diagnoses and better treatment regimens. -

A New Look at Transudation: the Apocrine Connection
Physiol. Res. 69: 227-244, 2020 https://doi.org/10.33549/physiolres.934229 REVIEW A New Look at Transudation: The Apocrine Connection Robert FARKAŠ1, Milan BEŇO1, Denisa BEŇOVÁ-LISZEKOVÁ1, Ivan RAŠKA2, Otakar RAŠKA2,3 1Laboratory of Developmental Genetics, Institute of Experimental Endocrinology, Biomedical Research Center, Slovak Academy of Sciences, Bratislava, Slovak Republic, 2Institute of Biology and Medical Genetics, First Faculty of Medicine, Charles University, Prague, Czech Republic, 3Institute of Pathophysiology, Third Faculty of Medicine, Charles University, Prague, Czech Republic Received June 9, 2019 Accepted December 20, 2019 Epub Ahead of Print March 23, 2020 Summary Corresponding authors Transcellular trafficking in which various molecules are Robert Farkaš, Laboratory of Developmental Genetics, Institute transported across the interior of a cell, is commonly classified as of Experimental Endocrinology, Biomedical Research Center, transcytosis. However, historically this term has been used Slovak Academy of Sciences, Dúbravská cesta 9, 84505 synonymously with transudation. In both cases transcellular Bratislava, Slovak Republic. E-mail: [email protected]. Otakar trafficking starts with the internalization of proteins or other Raška, Third Faculty of Medicine, Charles University, Ruská 87, compounds on the basal or basolateral side of a cell and 10000 Prague, Czech Republic. E-mail: [email protected] continues by their transport across the interior to the apical pole (or vice versa) where they are subsequently released. -

Volume 21 • 2015
Weill Co rnell Medical College Volume 21 • January 2015 The Newsletter of the Department of Pathology and Laboratory Medicine at NewYork-Presbyterian Hospital/Weill Cornell Medical Center Contents Research Highlights Localization of Annexin A5 on the surface of placental trophoblasts 1 by David P. Hajjar, PhD Research Highlights 2 Focus (green: AnxA5; blue: nucleus) Figure 1 : Expression of AnxA5 (green fluorescence) on 3 cultured syncytialized human placental trophoblasts. Clinical Pathology Update Blue (DAPI) fluorescence marks the nuclei. [bars = 50 m. Confocal microscopy 3-dimensional projections of Z-axis image stacks, voxel size ( m): width 0.73, height 0.73, and 4-6 Drs. Jacob Rand and Xiao-Xuan Wu. depth 1.50] (for complete description, see Wu XX, Guller S, The Department is delighted to welcome Dr. Jacob Rand , Rand JH. Hydroxychloroquine reduces binding of antiphos - Keynotes pholipid antibodies to syncytiotrophoblasts and restores Professor of Pathology and Laboratory Medicine, as Vice annexin A5 expression. Am J Obstet Gynecol . 2011 Dec; Chairman for Laboratory Medicine and Director of the Clinical 205(6):576.e7-14.) 7 Laboratories. Pathology Faculty’s Dr. Rand comes to us from Montefiore Medical Center and Global Travels the Albert Einstein College of Medicine where he served as Atomic force Microscopy: Structure of the Director of Hematology, Advanced Coagulation and Protein aPL Ig- 2GPI Complexes Separation Laboratories and as Professor of Pathology, 8 Medicine, and Obstetrics and Gynecology, and Director of the -HCQ Resident’s Corner Hematology Laboratories. Dr. Rand’s record of achievement spans across disciplines, with strong backgrounds as a clini cal physician, clinical laboratory director, teacher and 9-11 +HCQ basic scientist. -

(Eccrine and Apocrine Glands) During Childhood in Japanese, Especially on the PAS Positive Substance and Iron
Histochemical Investigation on the Axillary Sweat Glands (Eccrine and Apocrine Glands) during Childhood in Japanese, especially on the PAS Positive Substance and Iron By Iwao Yasui and Hiroshi Kagemoto Department of Anatomy, School of Medicine, Keio University Shinjuku, Tokyo, Japan (Director : Prof. Dr. T. Taniguchi) Introduction The application of periodic acid to histochemical study on poly- saccharide was made for the first time by Hot c h k i s s, M c Man u s, Lillie et G r e co and Marches e. This histochemical method was called for short PAS method " (namely, Periodic Acid S c h i f f). 'Recently , a good deal of the histochemical studies regarding glyco- gen, mucopolysaccharide and other substances in the sweat glands have been made by such various scholars as Bunt in g, Wislocki and Dempsey (1948), Montagna, Chase and Lobitz (1952), Montagna, Chase and M e l a r a g n o (1951) and others, apply- ing the PAS method. On the other hand, studies on the iron reaction in the sweat glands have been numerously reported since H o m m a (1925)'s research on the axillary sweat glands by Turnbull reaction, that is, Klaar (1926), Herzenberg (1927), Moriyama (1927), Oono and Kinoshita (1927), Wosllard (1930), Richter (1933), Manca (1934), Way and Memmesheimer (1938), Yoshi- mura (1942), Nagamitsu (1941), Yoshihiro (1942a), Cavaz- zana (1947), Bunting, Wislocki and Dempsey (1948),Iwa- shige (1951),Montagna,Chase and Lobitz (1953) and others. Nevertheless, there have been no reports which give histochemical observation on the axillary sweat glands during childhood by age group. -

Squamous Epithelium Are Thin, Which Allows for the Rapid Passage of Substances Through Them
Chapter 2 1st Prof. Anatomy Arsalan (Lecturer Department of Pharmacy University of Peshawar) Tissue is an aggregation of similar cells and their products that perform same function. There are four principal types of tissues in the body: ❑ epithelial tissue: covers body surfaces, lines body cavities and ducts and forms glands ❑ connective tissue: binds, supports, and protects body parts ❑ muscle tissue: produce body and organ movements ❑ nervous tissue: initiates and transmits nerve impulses from one body part to another • Epithelial tissues cover body and organ surfaces, line body cavities and lumina and forms various glands • Derived from endoderm ,ectoderm, and mesoderm • composed of one or more layers of closely packed cells • Perform diverse functions of protection, absorption, excretion and secretion. Highly cellular with low extracellular matrix Polar – has an apical surface exposed to external environment or body cavity, basal layer attached to underlying connective tissue by basement membrane and lateral surfaces attached to each other by intercellular junctions Innervated Avascular – almost all epithelia are devoid of blood vessels, obtain nutrients by diffusion High regeneration capacity Protection: Selective permeability: in GIT facilitate absorption, in kidney facilitate filtration, in lungs facilitate diffusion. Secretions: glandular epithelium form linings of various glands, involved in secretions. Sensations: contain some nerve endings to detect changes in the external environment at their surface Epithelium rests on connective tissue. Between the epithelium and connective tissue is present the basement membrane which is extracellular matrix made up of protein fibers and carbohydrates. Basement membrane attach epithelium to connective tissue and also regulate movement of material between epithelium and connective tissue Epithelial cells are bound together by specialized connections in the plasma membranes called intercellular junctions .