Federal Register/Vol. 85, No. 150/Tuesday, August 4, 2020/Notices
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Medical Review Officer Manual
Department of Health and Human Services Substance Abuse and Mental Health Services Administration Center for Substance Abuse Prevention Medical Review Officer Manual for Federal Agency Workplace Drug Testing Programs EFFECTIVE OCTOBER 1, 2010 Note: This manual applies to Federal agency drug testing programs that come under Executive Order 12564 dated September 15, 1986, section 503 of Public Law 100-71, 5 U.S.C. section 7301 note dated July 11, 1987, and the Department of Health and Human Services Mandatory Guidelines for Federal Workplace Drug Testing Programs (73 FR 71858) dated November 25, 2008 (effective October 1, 2010). This manual does not apply to specimens submitted for testing under U.S. Department of Transportation (DOT) Procedures for Transportation Workplace Drug and Alcohol Testing Programs (49 CFR Part 40). The current version of this manual and other information including MRO Case Studies are available on the Drug Testing page under Medical Review Officer (MRO) Resources on the SAMHSA website: http://www.workplace.samhsa.gov Previous Versions of this Manual are Obsolete 3 Table of Contents Chapter 1. The Medical Review Officer (MRO)........................................................................... 6 Chapter 2. The Federal Drug Testing Custody and Control Form ................................................ 7 Chapter 3. Urine Drug Testing ...................................................................................................... 9 A. Federal Workplace Drug Testing Overview.................................................................. -

House Bill No. 1176
FIRST REGULAR SESSION HOUSE BILL NO. 1176 101ST GENERAL ASSEMBLY INTRODUCED BY REPRESENTATIVE DAVIS. 2248H.01I DANA RADEMAN MILLER, Chief Clerk AN ACT To repeal sections 191.480 and 579.015, RSMo, and to enact in lieu thereof two new sections relating to investigational drugs. Be it enacted by the General Assembly of the state of Missouri, as follows: Section A. Sections 191.480 and 579.015, RSMo, are repealed and two new sections 2 enacted in lieu thereof, to be known as sections 191.480 and 579.015, to read as follows: 191.480. 1. For purposes of this section, the following terms shall mean: 2 (1) "Eligible patient", a person who meets all of the following: 3 (a) Has a debilitating, life-threatening, or terminal illness; 4 (b) Has considered all other treatment options currently approved by the United States 5 Food and Drug Administration and all relevant clinical trials conducted in this state; 6 (c) Has received a prescription or recommendation from the person's physician for an 7 investigational drug, biological product, or device; 8 (d) Has given written informed consent which shall be at least as comprehensive as the 9 consent used in clinical trials for the use of the investigational drug, biological product, or device 10 or, if the patient is a minor or lacks the mental capacity to provide informed consent, a parent or 11 legal guardian has given written informed consent on the patient's behalf; and 12 (e) Has documentation from the person's physician that the person has met the 13 requirements of this subdivision; 14 (2) "Investigational drug, biological product, or device", a drug, biological product, or 15 device, any of which are used to treat the patient's debilitating, life-threatening, or terminal 16 illness, that has successfully completed phase one of a clinical trial but has not been approved EXPLANATION — Matter enclosed in bold-faced brackets [thus] in the above bill is not enacted and is intended to be omitted from the law. -

21 CFR Ch. II (4–1–10 Edition) § 1308.12
§ 1308.12 21 CFR Ch. II (4–1–10 Edition) Some trade and other names: N,N- whenever the existence of such salts, Diethyltryptamine; DET isomers, and salts of isomers is possible (18) Dimethyltryptamine ................................................. 7435 Some trade or other names: DMT within the specific chemical designa- (19) 5-methoxy-N,N-diisopropyltryptamine (other tion: name: 5-MeO-DIPT) ................................................... 7439 (1) gamma-hydroxybutyric acid (some other names in- (20) Ibogaine .................................................................. 7260 clude GHB; gamma-hydroxybutyrate; 4- Some trade and other names: 7-Ethyl- hydroxybutyrate; 4-hydroxybutanoic acid; sodium 6,6b,7,8,9,10,12,13-octahydro-2-methoxy-6,9- oxybate; sodium oxybutyrate) .................................... 2010 methano-5H-pyrido [1′, 2′:1,2] azepino [5,4-b] (2) Mecloqualone ........................................................... 2572 indole; Tabernanthe iboga (3) Methaqualone ........................................................... 2565 (21) Lysergic acid diethylamide ..................................... 7315 (22) Marihuana .............................................................. 7360 (f) Stimulants. Unless specifically ex- (23) Mescaline ............................................................... 7381 cepted or unless listed in another (24) Parahexyl—7374; some trade or other names: 3- schedule, any material, compound, Hexyl-1-hydroxy-7,8,9,10-tetrahydro-6,6,9-trimethyl- 6H-dibenzo[b,d]pyran; Synhexyl. mixture, -

Phencyclidine: an Update
Phencyclidine: An Update U.S. DEPARTMENT OF HEALTH AND HUMAN SERVICES • Public Health Service • Alcohol, Drug Abuse and Mental Health Administration Phencyclidine: An Update Editor: Doris H. Clouet, Ph.D. Division of Preclinical Research National Institute on Drug Abuse and New York State Division of Substance Abuse Services NIDA Research Monograph 64 1986 DEPARTMENT OF HEALTH AND HUMAN SERVICES Public Health Service Alcohol, Drug Abuse, and Mental Health Administratlon National Institute on Drug Abuse 5600 Fishers Lane Rockville, Maryland 20657 For sale by the Superintendent of Documents, U.S. Government Printing Office Washington, DC 20402 NIDA Research Monographs are prepared by the research divisions of the National lnstitute on Drug Abuse and published by its Office of Science The primary objective of the series is to provide critical reviews of research problem areas and techniques, the content of state-of-the-art conferences, and integrative research reviews. its dual publication emphasis is rapid and targeted dissemination to the scientific and professional community. Editorial Advisors MARTIN W. ADLER, Ph.D. SIDNEY COHEN, M.D. Temple University School of Medicine Los Angeles, California Philadelphia, Pennsylvania SYDNEY ARCHER, Ph.D. MARY L. JACOBSON Rensselaer Polytechnic lnstitute National Federation of Parents for Troy, New York Drug Free Youth RICHARD E. BELLEVILLE, Ph.D. Omaha, Nebraska NB Associates, Health Sciences Rockville, Maryland REESE T. JONES, M.D. KARST J. BESTEMAN Langley Porter Neuropsychiatric lnstitute Alcohol and Drug Problems Association San Francisco, California of North America Washington, D.C. DENISE KANDEL, Ph.D GILBERT J. BOTV N, Ph.D. College of Physicians and Surgeons of Cornell University Medical College Columbia University New York, New York New York, New York JOSEPH V. -

Hallucinogen-Like Actions of 5-Methoxy-N,N-Diisopropyltryptamine in Mice and Rats ⁎ W.E
Pharmacology, Biochemistry and Behavior 83 (2006) 122–129 www.elsevier.com/locate/pharmbiochembeh Hallucinogen-like actions of 5-methoxy-N,N-diisopropyltryptamine in mice and rats ⁎ W.E. Fantegrossi a,b, , A.W. Harrington b, C.L. Kiessel b, J.R. Eckler c, R.A. Rabin c, J.C. Winter c, A. Coop d, K.C. Rice e, J.H. Woods b a Division of Neuroscience, Yerkes National Primate Research Center, Emory University, 954 Gatewood Road, Atlanta, GA 30322, United States b Department of Pharmacology, University of Michigan Medical School, Ann Arbor, MI, United States c Department of Pharmacology and Toxicology, School of Medicine and Biomedical Sciences, State University of New York at Buffalo, Buffalo, NY, United States d Department of Pharmaceutical Sciences, University of Maryland School of Pharmacy, Baltimore, MD, United States e Laboratory of Medicinal Chemistry, NIDDK, National Institutes of Health, Bethesda, MD Received 2 June 2005; received in revised form 7 December 2005; accepted 29 December 2005 Available online 3 February 2006 Abstract Few studies have examined the effects of 5-methoxy-N,N-diisopropyltryptamine (5-MeO-DIPT) in vivo. In these studies, 5-MeO-DIPT was tested in a drug-elicited head twitch assay in mice where it was compared to the structurally similar hallucinogen N,N-dimethyltryptamine (N,N- DMT) and challenged with the selective serotonin (5-HT)2A antagonist M100907, and in a lysergic acid diethylamide (LSD) discrimination assay in rats where its subjective effects were challenged with M100907 or the 5-HT1A selective antagonist WAY-100635. Finally, the affinity of 5- MeO-DIPT for three distinct 5-HT receptors was determined in rat brain. -

An Analysis of the Synthetic Tryptamines AMT and 5-Meo-DALT: Emerging “Novel Psychoactive Drugs”
Accepted Manuscript An Analysis of the Synthetic Tryptamines AMT and 5-MeO-DALT: Emerging “Novel Psychoactive Drugs” Warunya Arunotayanun, Jeffrey W. Dalley, Xi-Ping Huang, Vincent Setola, Ric Treble, Leslie Iversen, Bryan L. Roth, Simon Gibbons PII: S0960-894X(13)00394-6 DOI: http://dx.doi.org/10.1016/j.bmcl.2013.03.066 Reference: BMCL 20302 To appear in: Bioorganic & Medicinal Chemistry Letters Received Date: 31 January 2013 Revised Date: 18 March 2013 Accepted Date: 20 March 2013 Please cite this article as: Arunotayanun, W., Dalley, J.W., Huang, X-P., Setola, V., Treble, R., Iversen, L., Roth, B.L., Gibbons, S., An Analysis of the Synthetic Tryptamines AMT and 5-MeO-DALT: Emerging “Novel Psychoactive Drugs”, Bioorganic & Medicinal Chemistry Letters (2013), doi: http://dx.doi.org/10.1016/j.bmcl. 2013.03.066 This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain. Graphical Abstract To create your abstract, type over the instructions in the template box below. Fonts or abstract dimensions should not be changed or altered. An Analysis of the Synthetic Tryptamines AMT and 5- Leave this area blank for abstract info. MeO-DALT: Emerging “Novel Psychoactive Drugs” Warunya Arunotayanun, Jeffrey W. -
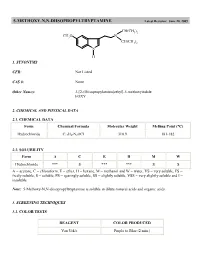
5-METHOXY-N,N-DIISOPROPYLTRYPTAMINE Latest Revision: June 20, 2005
5-METHOXY-N,N-DIISOPROPYLTRYPTAMINE Latest Revision: June 20, 2005 CH(CH3)2 CH3O N CH(CH3)2 N H 1. SYNONYMS CFR: Not Listed CAS #: None Other Names: 3-[2-(Diisopropylamino)ethyl]-5-methoxyindole FOXY 2. CHEMICAL AND PHYSICAL DATA 2.1. CHEMICAL DATA Form Chemical Formula Molecular Weight Melting Point (°C) Hydrochloride C17H27N2OCl 310.9 181-182 2.2. SOLUBILITY Form A C E H M W Hydrochloride *** S *** *** S S A = acetone, C = chloroform, E = ether, H = hexane, M = methanol and W = water, VS = very soluble, FS = freely soluble, S = soluble, PS = sparingly soluble, SS = slightly soluble, VSS = very slightly soluble and I = insoluble Note: 5-Methoxy-N,N-diisopropyltryptamine is soluble in dilute mineral acids and organic acids. 3. SCREENING TECHNIQUES 3.1. COLOR TESTS REAGENT COLOR PRODUCED Van Urk's Purple to Blue (2 min.) 3.2. THIN LAYER CHROMATOGRAPHY Visualization Van Urk's reagent Relative R COMPOUND f System TLC 18 dimethyltryptamine 0.32 diethyltryptamine 0.89 5-methoxy- -methyltryptamine 0.70 5-methoxydiisopropyltryptamine 1.0 (8.05 cm) 3.3. GAS CHROMATOGRAPHY Method DMT-GCS1 Instrument: Gas chromatograph operated in split mode with FID Column: J&W DB-1 15 m x 0.32 mm x 0.25 µm film thickness Carrier gas: Helium at 1.3 mL/min Temperatures: Injector: 275°C Detector: 280°C Oven program: 190°C for 10 min Injection Parameters: Split Ratio = 60:1, 1 µL injected Samples are to be dissolved in chloroform, washed with dilute sodium carbonate and filtered. COMPOUND RRT COMPOUND RRT indole 0.12 DET 0.37 MDA 0.15 C-4 phthalate 0.38 MDMA 0.17 5-MeO-AMT 0.42 tryptamine 0.23 5-MeODMT 0.46 AMT 0.24 DIPT 0.58 DMT 0.26 C-5 phthalate 0.65 caffeine 0.28 5-MeODIPT 1.00 (7.14 min) 4. -

Advisory Council on the Misuse of Drugs Chair: Professor Les Iversen Secretary: Rachel Fowler 3Rd Floor Seacole Building 2
ACMD Advisory Council on the Misuse of Drugs Chair: Professor Les Iversen Secretary: Rachel Fowler 3rd Floor Seacole Building 2. Marsham Street London SW1P 4DF 020 7035 0454 Email: [email protected] Rt Hon. Theresa May MP Home Office 2 Marsham Street London SW1P 4DF 18th October 2012 Dear Home Secretary, In March 2012 the ACMD advised that methoxetamine be subject to a temporary class drug order. Methoxetamine was marketed as a legal alternative to ketamine until a temporary class drug order was implemented in April 2012. As is now required, the ACMD has followed its initial assessment with a consideration of methoxetamine in the context of the Misuse of Drugs Act 1971; I enclose the report with this letter. The chemical structure of methoxetamine bears a close resemblance to that of both ketamine and phencyclidine (PCP, „Angel Dust‟, a class A drug), which both produce well- documented and serious adverse effects following both acute and chronic usage. Users report that the effects of methoxetamine are similar to those of ketamine, however, some users report that the effects are of longer duration.The harmful effects reported include severe dissociation, cardiovascular symptoms, paranoid thoughts and unpleasant hallucinations. The first analytically confirmed series reported by Guy‟s and St Thomas‟ NHS Foundation Trust, London in 2011, was of three individuals who presented having self-reported use of methoxetamine. All three presented with a ketamine-like dissociative state, but also had significant stimulant effects with agitation and cardiovascular effects including tachycardia and hypertension. Toxicological screening of serum samples confirmed methoxetamine use in two of the cases. -

Update of the Generic Definition for Tryptamines
ACMD Advisory Council on the Misuse of Drugs Chair: Professor Les Iversen Secretary: Zahi Sulaiman 2nd Floor (NW), Seacole Building 2 Marsham Street London SW1P 4DF Tel: 020 7035 1121 [email protected] Norman Baker MP, Minister for Crime Prevention Home Office 2 Marsham Street London SW1P 4DF 10 June 2014 Dear Minister, In December 2013, you commissioned the ACMD to begin a regular review of generic definitions under the Misuse of Drugs Act 1971, with the aim of capturing emerging new psychoactive substances. These drugs are variants of controlled drugs and fall outside the existing scope of the Misuse of Drugs Act 1971. The ACMD has considered evidence available on tryptamines in the context of the Misuse of Drugs Act 1971 and I enclose the Advisory Council’s advice and an expanded definition for tryptamine compounds with this letter. The ACMD’s NPS Committee has firstly reviewed previous research and existing controls to identify those tryptamines now seen to evade the existing controls. The ACMD has also reviewed data provided by the Home Office’s early warning systems and networks, clinical toxicology, prevalence and neuropharmacology in arriving at the expanded generic definition. This expanded generic definition will bring drugs such as alpha-methyltryptamine (AMT) as well as 5-MeO-DALT within the scope of the Misuse of Drugs Act 1971. These are highly potent hallucinogens which act on the 5HT2A receptor, in the same way as LSD. The ACMD therefore recommends that the tryptamines covered by the proposed expanded generic definition in this report, are controlled under the Misuse of Drugs Act (1971) as Class A substances. -

Application of High Resolution Mass Spectrometry for the Screening and Confirmation of Novel Psychoactive Substances Joshua Zolton Seither [email protected]
Florida International University FIU Digital Commons FIU Electronic Theses and Dissertations University Graduate School 4-25-2018 Application of High Resolution Mass Spectrometry for the Screening and Confirmation of Novel Psychoactive Substances Joshua Zolton Seither [email protected] DOI: 10.25148/etd.FIDC006565 Follow this and additional works at: https://digitalcommons.fiu.edu/etd Part of the Chemistry Commons Recommended Citation Seither, Joshua Zolton, "Application of High Resolution Mass Spectrometry for the Screening and Confirmation of Novel Psychoactive Substances" (2018). FIU Electronic Theses and Dissertations. 3823. https://digitalcommons.fiu.edu/etd/3823 This work is brought to you for free and open access by the University Graduate School at FIU Digital Commons. It has been accepted for inclusion in FIU Electronic Theses and Dissertations by an authorized administrator of FIU Digital Commons. For more information, please contact [email protected]. FLORIDA INTERNATIONAL UNIVERSITY Miami, Florida APPLICATION OF HIGH RESOLUTION MASS SPECTROMETRY FOR THE SCREENING AND CONFIRMATION OF NOVEL PSYCHOACTIVE SUBSTANCES A dissertation submitted in partial fulfillment of the requirements for the degree of DOCTOR OF PHILOSOPHY in CHEMISTRY by Joshua Zolton Seither 2018 To: Dean Michael R. Heithaus College of Arts, Sciences and Education This dissertation, written by Joshua Zolton Seither, and entitled Application of High- Resolution Mass Spectrometry for the Screening and Confirmation of Novel Psychoactive Substances, having been approved in respect to style and intellectual content, is referred to you for judgment. We have read this dissertation and recommend that it be approved. _______________________________________ Piero Gardinali _______________________________________ Bruce McCord _______________________________________ DeEtta Mills _______________________________________ Stanislaw Wnuk _______________________________________ Anthony DeCaprio, Major Professor Date of Defense: April 25, 2018 The dissertation of Joshua Zolton Seither is approved. -

N,N-Dimethyltryptamine Compound Found in the Hallucinogenic Tea Ayahuasca, Regulates Adult Neurogenesis in Vitro and in Vivo Jose A
Morales-Garcia et al. Translational Psychiatry (2020) 10:331 https://doi.org/10.1038/s41398-020-01011-0 Translational Psychiatry ARTICLE Open Access N,N-dimethyltryptamine compound found in the hallucinogenic tea ayahuasca, regulates adult neurogenesis in vitro and in vivo Jose A. Morales-Garcia 1,2,3,4, Javier Calleja-Conde 5, Jose A. Lopez-Moreno 5, Sandra Alonso-Gil1,2, Marina Sanz-SanCristobal1,2, Jordi Riba6 and Ana Perez-Castillo 1,2,4 Abstract N,N-dimethyltryptamine (DMT) is a component of the ayahuasca brew traditionally used for ritual and therapeutic purposes across several South American countries. Here, we have examined, in vitro and vivo, the potential neurogenic effect of DMT. Our results demonstrate that DMT administration activates the main adult neurogenic niche, the subgranular zone of the dentate gyrus of the hippocampus, promoting newly generated neurons in the granular zone. Moreover, these mice performed better, compared to control non-treated animals, in memory tests, which suggest a functional relevance for the DMT-induced new production of neurons in the hippocampus. Interestingly, the neurogenic effect of DMT appears to involve signaling via sigma-1 receptor (S1R) activation since S1R antagonist blocked the neurogenic effect. Taken together, our results demonstrate that DMT treatment activates the subgranular neurogenic niche regulating the proliferation of neural stem cells, the migration of neuroblasts, and promoting the generation of new neurons in the hippocampus, therefore enhancing adult neurogenesis and -

Model Scheduling New/Novel Psychoactive Substances Act (Third Edition)
Model Scheduling New/Novel Psychoactive Substances Act (Third Edition) July 1, 2019. This project was supported by Grant No. G1799ONDCP03A, awarded by the Office of National Drug Control Policy. Points of view or opinions in this document are those of the author and do not necessarily represent the official position or policies of the Office of National Drug Control Policy or the United States Government. © 2019 NATIONAL ALLIANCE FOR MODEL STATE DRUG LAWS. This document may be reproduced for non-commercial purposes with full attribution to the National Alliance for Model State Drug Laws. Please contact NAMSDL at [email protected] or (703) 229-4954 with any questions about the Model Language. This document is intended for educational purposes only and does not constitute legal advice or opinion. Headquarters Office: NATIONAL ALLIANCE FOR MODEL STATE DRUG 1 LAWS, 1335 North Front Street, First Floor, Harrisburg, PA, 17102-2629. Model Scheduling New/Novel Psychoactive Substances Act (Third Edition)1 Table of Contents 3 Policy Statement and Background 5 Highlights 6 Section I – Short Title 6 Section II – Purpose 6 Section III – Synthetic Cannabinoids 13 Section IV – Substituted Cathinones 19 Section V – Substituted Phenethylamines 23 Section VI – N-benzyl Phenethylamine Compounds 25 Section VII – Substituted Tryptamines 28 Section VIII – Substituted Phenylcyclohexylamines 30 Section IX – Fentanyl Derivatives 39 Section X – Unclassified NPS 43 Appendix 1 Second edition published in September 2018; first edition published in 2014. Content in red bold first added in third edition. © 2019 NATIONAL ALLIANCE FOR MODEL STATE DRUG LAWS. This document may be reproduced for non-commercial purposes with full attribution to the National Alliance for Model State Drug Laws.