Method of Inhibiting Acrylamide Formation and Use Thereof
Total Page:16
File Type:pdf, Size:1020Kb

Load more
Recommended publications
-

Noriedgewatertogo2018-10.Pdf
Shoyu Ramen 12.95 Nori Appetizers Nori Salads Japanese Egg Noodles, Soy Sauce Pork Broth, Pork Chashu, Nori Kitchen Entrees Sushi Vegetable Maki Some of our dishes may contains sesame seeds and seaweed. Marinated Bamboo Shoots, Quail Eggs, Spinach, Japanese Served with Miso Soup, Salad and Rice (add $1 for spicy miso soup) Nigiri (Sliced fish on a bed of rice 1 pc/ order) Contains Sesame Seeds (add $1 for soy paper) Some of our dishes may contains sesame seeds and seaweed. Some of Our Maki Can Be Made in Hand Roll Style. Edamame 5.00 Mixed Greens Salad 6.00 Prepared Vegetables and Sesame Seeds. Topped with Green Sashimi (Sliced fish) (2 pcs./ order) add $2.00 Steamed Japanese Soy Bean, Sprinkled Lightly with Salt. Mixed Greens, Carrots, Cucumbers and Tomatoes. Served With Onion, Fish Cake and Seaweed Sheet. Shrimps & Vegetables Tempura Dinner 13.95 Ebi Avocado Maki Avocado. 4.00 Botan Ebi sweet shrimp with fried head 3.50 Nori Tempura (2 Shrimps 6 Vegetables)9.50 Japanese Style Ginger Dressing Or Creamy Homemade Dressing. Chicken Katsu Ramen 12.95 Deep Fried Shrimps and Vegetables Tempura Avocado Tempura Maki 6.00 Deep Fried Shrimps and Assorted Vegatables; Sunomono Moriawase (Japanese Seafood Salad) 9.00 Japanese Egg Noodles, Marinated Bamboo Shoots, Quail Eggs, with Tempura Sauce. Ebi boiled shrimp 2.50 Avocado tempura with spicy mayo. Crab meat, Ebi, Crab Stick, Tako, Seaweed and Cucumber A Quintessentially Japanese Appetizer. Spinach, Japanese Prepared Vegetables and Sesame Seeds. Chicken Katsu 13.95 Escolar super white tuna 3.50 Asparagus Maki Steamed asparagus. -

Menu Ake Wehpo Yleosuo Gmoein Sgta Artll T Do Ayyo!Ur Day!
BANGKOK SUKHUMVIT E ats & treats Restaurant Menu AKe wehpo yleosuo gmoein sgta artll t do ayyo!ur day! THB 180 Breakfast Fresh Fruit Salad Set Breakfast Seasonal Fruit Compote THB 180 Your choice of apple, peach or prune Continental Breakfast THB 420 Yoghurt THB 160 Your choice of juice: Orange, Pineapple, Apple, Low Fat Yoghurt Plain Yoghurt Mixed Fruit Yoghurt Guava or Tomato Your choice of morning breakfast pastries (3 pieces) Morning Pastries Choose from: Plain Croissant, Whole Wheat Croissant, Selection of 3 Pieces per Serving THB 160 Chocolate Danish, Fruit Danish, Doughnut, Chocolate Selection of 5 pieces per Serving THB 200 Muffin, Low Fat Muffin, Soft Rolls, Hard Rolls, French Baguette, Whole Wheat Toast or Regular Toast Plain Croissant Doughnut Whole wheat croissant Served with choose 3: Butter, Margarine, Salted Butter, Low Fat Muffin Fruit Danish Chocolate Danish Strawberry Jam, Pineapple Jam, Orange Marmalade, Plain Toast Soft Roll Chocolate Muffin Nutella, Peanut Butter, Honey Plain Toast French Baguette Whole Wheat toast Seasonal Fresh Fruit Plate Served with choose 3 Or Fruit-Flavored or Natural Low Fat Yoghurt Butter Margarine Pineapple Jam Salted butter Nutella Strawberry Jam Freshly Brewed Tea or Coffee, Decaffeinated Coffee or Hot Chocolate Honey Peanut Butter Orange Marmalade Cereals THB 180 American Breakfast THB 480 Corn Flakes Rice Krispies Coco Pop Your choice of Continental Breakfast Muesli All bran And 2 Eggs Cooked to Your Liking: Fried, Poached, Scrambled, Boiled or Omelette Served with choice of : Served with -

(12) Patent Application Publication (10) Pub. No.: US 2006/0194743 A1 Oku Et Al
US 2006O1947.43A1 (19) United States (12) Patent Application Publication (10) Pub. No.: US 2006/0194743 A1 Oku et al. (43) Pub. Date: Aug. 31, 2006 (54) METHOD FOR INHIBITING ACRYLAMIDE Publication Classification FORMATION AND USE THEREOF (51) Int. Cl. A6II 3 L/70 (2006.01) (76) Inventors: Kazuyuki Oku, Okayama (JP); Michio A6II 3L/98 (2006.01) Kubota, Okayama (JP); Shigeharu A2.3L 3/05 (2006.01) Fukuda, Okayama (JP); Toshio (52) U.S. Cl. ............................. 514/23: 514/561; 426/665 Miyake, Okayama (JP) (57) ABSTRACT Correspondence Address: An object of the present invention is to establish a method BROWDY AND NEIMARK, P.L.L.C. for actively suppressing the formation of acrylamide, which 624 NINTH STREET, NW is formed by heating edible materials at a relatively high temperature, and uses thereof, and to provide more safe SUTE 3OO compositions such as foods, beverages, cosmetics, pharma WASHINGTON, DC 20001-5303 (US) ceuticals, and intermediates thereof. The present invention solves the above object by providing: a method for Sup (21) Appl. No.: 10/536,268 pressing the formation of acrylamide, comprising the steps of incorporating an organic Substance having an ability of (22) PCT Filed: Nov. 20, 2003 Suppressing the formation of acrylamide into an edible material having a possibility of forming acrylamide by (86). PCT No.: PCT/UP03/14819 heating and heating the resulting mixture, when heating the edible material; compositions such as foods, beverages, (30) Foreign Application Priority Data cosmetics, pharmaceuticals, and intermediates thereof, pro duced by the method, whose possibility of forming acryla Nov. 27, 2002 (JP)..................................... -

Favorite Foods of the World.Xlsx
FAVORITE FOODS OF THE WORLD - VOTING BRACKETS First Round Second Round Third Round Fourth Round Sweet Sixteen Elite Eight Final Four Championship Final Four Elite Eight Sweet Sixteen Fourth Round Third Round Second Round First Round Votes Votes Votes Votes Votes Votes Votes Votes Votes Votes Votes Votes Votes Votes Votes Votes Blintzes Duck Confit Papadums Laksa Jambalaya Burrito Cornish Pasty Bulgogi Nori Torta Vegemite Toast Crepes Tagliatelle al Ragù Bouneschlupp Potato Pancakes Hummus Gazpacho Lumpia Philly Cheesesteak Cannelloni Tiramisu Kugel Arepas Cullen Skink Börek Hot and Sour Soup Gelato Bibimbap Black Forest Cake Mousse Croissants Soba Bockwurst Churros Parathas Cream Stew Brie de Meaux Hutspot Crab Rangoon Cupcakes Kartoffelsalat Feta Cheese Kroppkaka PBJ Sandwich Gnocchi Saganaki Mochi Pretzels Chicken Fried Steak Champ Chutney Kofta Pizza Napoletana Étouffée Satay Kebabs Pelmeni Tandoori Chicken Macaroons Yakitori Cheeseburger Penne Pinakbet Dim Sum DIVISION ONE DIVISION TWO Lefse Pad Thai Fastnachts Empanadas Lamb Vindaloo Panzanella Kombu Tourtiere Brownies Falafel Udon Chiles Rellenos Manicotti Borscht Masala Dosa Banh Mi Som Tam BLT Sanwich New England Clam Chowder Smoked Eel Sauerbraten Shumai Moqueca Bubble & Squeak Wontons Cracked Conch Spanakopita Rendang Churrasco Nachos Egg Rolls Knish Pastel de Nata Linzer Torte Chicken Cordon Bleu Chapati Poke Chili con Carne Jollof Rice Ratatouille Hushpuppies Goulash Pernil Weisswurst Gyros Chilli Crab Tonkatsu Speculaas Cookies Fish & Chips Fajitas Gravlax Mozzarella Cheese -

The Word “Sampan” Comes from the Original Cantonese Term Used for the Boats, Which Literally Means “Three Planks”
The word “Sampan” comes from the original Cantonese term used for the boats, which literally means “three planks”. It popularly refers to a flat-bottomed, eloquent wooden boat gliding down the tranquil waterways laden with significant things of life. A sight which is very commonly visible in Asian lands, these boats appear to be sacred against the mesmerizing backdrop of nature and rhythmic sound of chopping of Yulah…the oars. More than a lifeline to the hard working natives of these lands, the boats are a symbol of “Simplicity”. Inspired by these sampans, our celebrated Pan- Asian restaurant Sampan, located atop the magnificent façade at The Suryaa, New Delhi is a confluence of simplicity, traditions, exotic flavors of food and aesthetics of the land. Offering a breath-taking view of New Delhi’s skyline, the restaurant features natural aroma of the spices, wholesome goodness in every morsel of the honest food, delicately prepared by our skilful chefs with a contemporary touch and served with warmth and affection-is the real story of sampan. Please let your server know if you have any special dietary requirements like Sugar free, low calorie, low sodium food and any food allergies and intolerances. Government taxes as applicable. We levy no service charge. Hotness Level Vegetarian Non – Vegetarian Healthy Signature SUSHI SASHIMI Nigiri Sushi (Two pieces per portion) 545 A slice of your choice of topping, hand pressed onto a mound of delicate vinegar rice, enhanced with a dash of wasabi Maguro Tuna Shake Salmon Ebi Prawn Tobikko Flying -
NOVEMBER 2020 Page
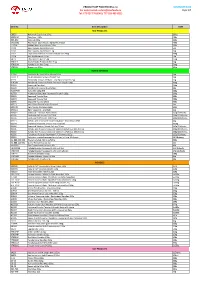
PRODUCT LIST TAKO FOODS s.r.o NOVEMBER 2020 For orders email: [email protected] Page 1/7 Tel: 773 027 776 (ENG), 777 926 495 (CZE) Item No. Item Description UoM RICE PRODUCTS 08930 Hakumaki Sushi rice 10 kg 10 kg 303013A Rice flour 400g 400g 839-840 Shiokoji 220g 220g CH16032 Rice Paper 22cm Round Spring-Roll 400g 400g J1371A Malted Rice - Kome Kouji 200g 200g J3100 Rice Toyama Koshihikari 5kg 5kg J3101 Rice Toyama Koshihikari 1 kg 1kg J3102 Toyama Koshihikari Funwari Gohan rice 200g 200g J3104 Rice Akitakomachi 5 kg 5kg J4075 Thai Jasmine Rice 25 kg 25kg YTK019 Koshi Yutaka Premium Rice 5 kg 5kg YTK023G Yutaka Sushi Rice 500g 500g YTK033 Brown rice 10kg 10kg NORI & SEAWEED 17164 Dashi Konbu Dried Kelp (Akaya) 1 kg 1kg CN-11-1 Chuka Wakame Seaweed salad 1kg 1kg K1131 Yamanaka Tokuyo Mehijiki - Dry Hijiki Seaweed 25g 25g K1450B Powdered Seaweed Aonori Premium Grade 100g 100g K1619 Seaweed Ogo Nori 500g K1628 Shredded Seaweed Kizami Nori 50g K1697WR Kaiso Mix 100g WR 100g K1703 Ariake Yaki Bara Nori Seaweed Grade A 100g 100g K2903 Seaweed Tosaka Blue 500g K2904 Seaweed Tosaka Red 500g K2905 Seaweed Tosaka White 500g K3214 Kelp Yama Dashi Konbu (Sokusei) 1kg K3217B Kelp Ma Konbu (Hakodate) 500g K3261A Hijiki Seawood - Me Hijiki 1kg K33021 Seaweed - Yakinori Aya Full Size 125g/50sheets K3305 Sushi nori full sheets 100/230G 230g/100sheets K3306 Sushi nori half sheets 100/125g 125g/100sheets K3313 Kofuku nori Seasoned seaweed Ajitsuke Nori Ohavo 8Pkt 24g K3314 Seasoned seaweed sesame nori chips 8G 30/8g K3340 Seaweed Yakinori Miyabi full -

JAPANESE FOOD CULTURE Enjoying the Old and Welcoming the New
For more detailed information on Japanese government policy and other such matters, see the following home pages. Ministry of Foreign Affairs Website http://www.mofa.go.jp/ Web Japan http://web-japan.org/ JAPANESE FOOD CULTURE Enjoying the old and welcoming the new Rice The cultivation and consumption of rice has always played a central role in Japanese food culture. Almost ready for harvesting, this rice field is located near the base of the mountain Iwakisan in Aomori Prefecture. © Aomori prefecture The rice-centered food culture of Japan and imperial edicts gradually eliminated the evolved following the introduction of wet eating of almost all flesh of animals and fowl. rice cultivation from Asia more than 2,000 The vegetarian style of cooking known as years ago. The tradition of rice served with shojin ryori was later popularized by the Zen seasonal vegetables and fish and other marine sect, and by the 15th century many of the foods products reached a highly sophisticated form and food ingredients eaten by Japanese today Honzen ryori An example of this in the Edo period (1600-1868) and remains had already made their debut, for example, soy formalized cuisine, which is the vibrant core of native Japanese cuisine. In sauce (shoyu), miso, tofu, and other products served on legged trays called honzen. the century and a half since Japan reopened made from soybeans. Around the same time, © Kodansha to the West, however, Japan has developed an a formal and elaborate incredibly rich and varied food culture that style of banquet cooking includes not only native-Japanese cuisine but developed that was derived also many foreign dishes, some adapted to from the cuisine of the Japanese tastes and some imported more or court aristocracy. -

SUSHI & MAIN COURSES – English Sushi Menu
SUSHI & MAIN COURSES – English Sushi Menu Tairyo sushi menu (Chef’s selection) Geisha 6 pcs: 4nigiri 2maki 65:- Samurai 9 pcs: 6nigiri 3maki 95:- Shogun 13 pcs: 9 nigiri 4maki 125:- Shake 9 pcs: 9 salmon nigiri 120:- Sumo 17 pcs: 12nigiri 5maki 180:- Vego-mix 10 pcs: 10 vego nigiri 95:- Family sushi 30 pcs: 20nigiri 10maki 260:- Combo: small sashimi and 6 pcs nigiri 180:- Fifty Fifty 10 pcs: 5salmon 5shrimps 130:- Chirashi Sushi 110:- Sashimi large 160:- Sashimi small 105:- Sashimi salad 105:- Tairyo norimaki (seaweedrolls) Falun Roll: chilimarinated tuna, mayo, avo, cucumber 110:- Tempura Roll: tempuraebi, avocado, mayo, scallions, salad and tempurasauce 120:- California Roll: krabba, avo, omelet, mayo, cucumber 85:- Spicy Tuna Roll: tuna, onion, cucumber, chilipaste, 6 pcs 95:- Philadelphia Roll: salmon, mayo, avo, red onion, philadelphia 105:- Kenzo Roll vegetarian 85:- Futomaki: salmon, cucumber, crab 85:- Maki mix 10 pcs: mixture av norimaki 105:- Tariyo roll of the day: chef’s selection 120:- Kappamaki: cucumberroll, 6 pcs 40:- Shakemaki: salmonroll, 6 pcs 45:- Tekkamaki: tunaroll, 6 pcs 50:- Gunkan: filled seaweedboat 2st 40:- Temaki: seaweedcone 40:- New! Rainbow Roll 130:- Tairyo main courses Yakiniku: Sliced beef with yakinikusauce 110:- Yakitori: Grilled chicken on spit with sauce of the house 110:- Bento: Yakiniku, yakitori, 5 pcs sushi 130:- Unagi: Grilled eel with unagi sauce 120:- Tempura: Friede shrimps done japanese style 120:- Karaage: Fried chicken done japanese style 130:- Ramen: Japanese noodle soup 110:- New! Gyoza: -

Sushi: a Global Love a Air
2019 ₈FEB Sushi: A Global Love Aair INSIDE: Seattle’s Sushi Story A phoenix fantastically painted with a “Taka-makie” technique (lacquerwork in which multiple layers of “Makie” create a beautiful, embossed design). In order to bring the divine phoenix to life, genuine gold “Makie” designs were boldly layered and subtle attention was paid to every single feather. Special lacquering techniques such as “Nashiji” and “Raden” were lavishly used on details bringing the gorgeous art of Urushi to life. Traditional Japanese Art ––––––– Handcrafted Urushi Lacquerware EXCLUSIVE U.S. DISTRIBUTOR OF WORLD-RENOWNED YAMADA HEIANDO LACQUERWARE ––––––– www.heiandoamerica.com [email protected] JAPONESSA_OrigamiMag_fullpage_001.pdf 1 2/17/18 9:39 PM C M Y CM MY CY CMY K MODERN JAPANESE CUISINE WITH A 1400 1ST AVE SEATTLE LATIN FLAIR 500 BELLEVUE WAY NE SUITE 130 BELLEVUE HAPPY HOURS DAILY 2019 FEB PUBLISHER’S NOTE IN JAPAN, we say “New Year’s Day is the key of the year.” ₈contents As a cultural bridge between the US and Japan, we are planning to expand Origami to the online platform and to other cities so Americans will learn even more about Japan. We are preparing online content that will make you want Sushi: A Global Love Aairto dive into Japan. Our online offerings will include information that could not be included in the magazine. We want to introduce a more interesting and unique FEATURE Sushi: A Global Love Affair “Japan.” The site will be full of rarely seen Japanese cuisine, 2 dances, events, and much more. Don’t miss it! Now that we have raised your expectations for Origami, here is some big news: More direct flights between Seattle and Japan will become available in 2019. -

Volume 16, Number 2 (2020)
Volume 16, No. 2 (2020) Asia Pacific Perspectives Volume 16,Imperial Number Bovine Bodies 2 -(2020) Mitsuda • 1 Volume 16, Number 2 (2020) CENTER FOR ASIA PACIFIC STUDIES EDITORIAL BOARD Dr. Melissa Dale Miriam Gross, University of Oklahoma Editor, Executive Director ann-elise lewallen, University of California, Santa Neha Cariappa Barbara Editorial Assistant John Nelson, University of San Francisco Ryoko Yamamoto, SUNY Westbury CONTENTS EDITOR’S INTRODUCTION Dr. Melissa Dale ............................................................................. 2 ARTICLES Imperial Bovine Bodies: Rendering Chinese Milk and Meat Fit for German and Japanese Consumption Tatsuya Mitsuda ............................................................................. 4 THINK PIECES Known Unknowns in Japanese Food History Eric C. Rath ................................................................................ 34 Diaspora, Exclusion and Appropriation: The Cuisine of the Korean Minority in Japan Christopher Laurent ......................................................................... 48 PHOTO ESSAY Contemporary Filipino Foodways: Views from the Street, Household, and Local Dining Ty Matejowsky ............................................................................. 67 GRADUATE STUDENT PAPER Blurred Boundaries between Food and Medicine: Traditional Chinese Medicine and Its Impact on Contemporary Chinese Self-Care Xiaoyu (Jennifer) Zhang ..................................................................... 78 BOOK REVIEW Fu, Jia-Chen. The Other -

Signature Cuts
DINNER MENU FUTAGO25USA.COM APPETIZER SIGNATURE CUTS ASSORTED KIMCHI $16 DRAGON KALBI $38 ASSORTED NAMUL $16 HARAMI STEAK $35 SPINACH, FERN, DAIKON & CARROT, SPROUT THICKCUT BEEF TONGUE $30 EDAMAME $6 THICKCUT TENDERLOIN STEAK $38 IPPON HORMON $25 YAKINORI SEAWEED $4 FRENCH FRIES WITH TRUFFLE SALT $9 SPICY CRISPY "BONCHOL" CHICKEN $12 TRADITIONAL YAKINIKU CUTS SHRIMP AND QUAIL EGG & SHISO $5 PRIME SHORT RIB KALBI $30 BEEF YUKHOE TARTARE $25 PRIME SKIRT STEAK HARAMI $28 A5 WAGYU SIRLOIN CARPACCIO BLACK TRUFFLE $55 TENDERLOIN $30 GRILLED MUSHROOM & BUTTER IN FOIL $20 BEEF TONGUE $28 TONTORO PORK NECK $18 KUROBUTA BERKSHIRE PORK KALBI $20 SALAD BERKSHIRE PORK SAUSAGE $14 HORMON LARGE INTESTINE $18 FUTAGO CHOREGI SALAD $13 MINO 1ST STOMACH $20 SPINACH GARLIC SALAD $13 SPICY CHICKEN $16 GOMASHIO CABBAGE $13 SANGCHU LETTUCE $9 VEGETABLES ONION $8 CORN $8 ASPARAGUS $8 BEEF SUSHI (1 PIECE) SHIITAKE MUSHROOM $10 $20 *2 PIECE MINIMUM PER ORDER ASSORTED VEGETABLES GARLIC IN SESAME OIL $8 WAGYU SPECIAL TORO $14 UNI AND WAGYU $17 RICE, NOODLES AND SOUP FOIE GRAS AND FILLET $14 LIMITED SPECIAL WAGYU & UNI JU $78 ASSORTED BEEF SUSHI $39 WAGYU SPECIAL TORO, UNI AND WAGYU, FOIE GRAS AND FILLET HOT STONE GARLIC RICE $16 HOT STONE BIBIMBAP $18 JAPANESE STEAMED RICE $5 BONEMARROW SPICY SOUP $15 BONEMARROW VEGETABLE SOUP $14 WAGYU BONEMARROW EGG SOUP $12 JAPANESESTYLE COLD REIMEN NOODLES $8 LIMITED HAMIDERU KALBI MP TRADITIONAL JAPANESE SOUL FOOD LIMITED TREASURE BOX MP 10 SECONDS WAGYU SIRLOIN ONE SLICE $26 DESSERT LIGHTLY SEARED WAGYU WITH UNI & SHISO $58 BLACK WAGYU KALBI $68 THE ORIGINAL GOLD RUSH $12 AZUKI MATCHA RUSH $12 FUTAGO MATCHA TIRAMISU $10 Items marked in red indicate our signature and most popular dishes. -

WE CATER! 8321 Lincoln Blvd [email protected] Happy People
≤ Simple Food. WE CATER! 8321 Lincoln Blvd [email protected] Happy People. Los Angeles, CA 90045 Humble Lasts, 323.989.BAGA(2242) Hunger Shouldn’t. [email protected] Where there’s good will, There’s good eats. Store Hours Eat Well. Feel Good. M-F: 11:30a - 10p Live Humbly. Sat-Sun: 12p - 10p BURGERS SANDWICHES RICE PLATES Hambága: Humble ingredients, Perfect Balance! Koke Kokko: Krazy Good! Karê Loco Moco: Have a Feast and Take a Nap! Angus patty, caramelized onion, garlic jam, romaine, House ground grilled chicken patty, ginger, garlic-spice, Double Angus patty, slow-cooked Japanese curry, fried tomato, HP sauce tomato, yuzu-jalapeño slaw, sunny egg, HP sauce egg Karê-bága: Comforting Soul Food Yakiniku Sando: HP-Inspired Philly Steak Chicken Katsu with Karê: Classic Comfort Angus patty, slow cooked Japanese curry, tomato, Sliced rib-eye steak, Yakiniku marinade, grilled Panko-crusted fried chicken, slow-cooked Japanese curry yuzu-jalapeño slaw, sunny egg onion, scallion, shichimi togarashi, garlic-butter roll * Add fried egg * Add cheese Tempeh-bága: Meaty Veggie. Yakiniku Plate: Our Take on Traditional Gyu-don Organic tempeh, caramelized onion, provolone melt, The B.A.T Bird: Bacon. Avocado. Tomato Sliced rib-eye steak, Yakiniku marinade, grilled onion, garlic jam, avocado, romaine, tomato, HP sauce Grilled chicken breast, melted cheddar, LOTS of bacon, avocado, scallion, Shichimi Togarashi tomato, creamy yuzu-vinaigrette, toasted roll * Add fried egg Jack’D: Hit the Road! *Substitute grilled chicken with chicken katsu Angus