[EC 1.4.3.4. (MAO)] Is a Flavin Adeni
Total Page:16
File Type:pdf, Size:1020Kb

Load more
Recommended publications
-

Enzyme2 File
Enzymes Theories of enzyme substrate interaction 2 Theories of enzyme substrate interaction . There are two proposed methods by which enzymes bind to their substrate molecules: 1- Lock & key model 2-Induced fit model 3 Theories of enzyme substrate interaction 1-lock & key model (Template hypothesis for enzyme action): The substrate fits to binding site of enzyme as a key fits into proper lock . Enzyme & substrate possess specific complementary geometric & rigid shapes fit exactly into one another. This explains enzyme specificity, but fails to explain the stabilization of the transition state & allosteric regulation & inhibition. 4 1-lock & key model : 5 Theories of enzyme substrate interaction 2-Induce fit model More flexible model than lock & key model. Enzymes are flexible structures The active site modified as the [S] interacts with the anzyme (active site of enzyme become complementary to S) Model explains enzyme specificty & stabilization of transition state, regulation & inhibition. 6 2-Induce fit model 7 Enzyme Inhibition 8 Enzyme inhibitors: Any substance that can diminish the velocity of an enzyme-catalyzed reaction. 9 Types of inhibition Irreversible Reversible inhibitors inhibitors 10 Types of inhibition 1-Irreversible inhibitors : Bind to enzymes through covalent bond (inhibited enzyme does not regain activity upon dilution of enzyme-inhibitor complex) 11 Type of inhibition 2-Reversible inhibitors: Bind to enzymes through noncovalent bonds, thus dilution of the enzyme–inhibitor complex results in dissociation of the reversibly bound inhibitor, and recovery of enzyme activity. 12 Reversible inhibitors 1. Competitive inhibition 2. Non competitive inhibition 3. Uncompeptitive inhibition 13 1-Competitive inhibition The inhibitor and substrate compete for the same active site on the enzyme as a result of similarity in structure. -
BI/CH421 Biochemistry I Last Name Or Initials: ______Exam 3 11/10/2014 Last Name (PRINT)
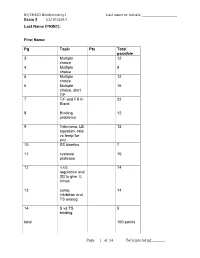
BI/CH421 Biochemistry I Last name or initials: ________________________ Exam 3 11/10/2014 Last Name (PRINT): First Name: Pg Topic Pts Total possible 3 Multiple 12 choice 4 Multiple 9 choice 5 Multiple 12 choice 6 Multiple 16 choice, start T/F 7 T/F and Fill in 22 Blank 8 Binding 12 problems 9 Yakimima, LB 12 equation, rate vs temp for enz 10 SS kinetics 7 11 cysteine 15 protease 12 ∆∆G, 14 regulation and [S] to give ¾ Vmax 13 comp. 14 inhibition and TS analog 14 S vs TS 5 binding total 150 points Page of 11 4 Total pts for pg _________ BI/CH421 Biochemistry I Last name or initials: ________________________ Exam 3 11/10/2014 Instructions: READ INSTRUCTIONS BEFORE BEGINNING EXAM. 1) Carefully read question before answering. Often I highlight very important information so please make note when I do so to make sure you are answering the question correctly. 2) Write your FULL name above and at least your last name or initials on every page. 3) Write all of your answers on the exam paper itself in the space provided. If you need additional space, you can write on the back of the SAME page. If you do this, you must write “ON BACK” so that we know where to look for your answer. 4) Your answers should be brief and legible. A correct answer that cannot be read cannot receive full credit. Additionally, extremely lengthy responses containing both correct and incorrect statements will be graded accordingly. Meaning, if you answer the question correctly but if you go on to write a “kitchen sink” response containing incorrect information, you will not receive full credit for that answer. -

Understanding Drug-Drug Interactions Due to Mechanism-Based Inhibition in Clinical Practice
pharmaceutics Review Mechanisms of CYP450 Inhibition: Understanding Drug-Drug Interactions Due to Mechanism-Based Inhibition in Clinical Practice Malavika Deodhar 1, Sweilem B Al Rihani 1 , Meghan J. Arwood 1, Lucy Darakjian 1, Pamela Dow 1 , Jacques Turgeon 1,2 and Veronique Michaud 1,2,* 1 Tabula Rasa HealthCare Precision Pharmacotherapy Research and Development Institute, Orlando, FL 32827, USA; [email protected] (M.D.); [email protected] (S.B.A.R.); [email protected] (M.J.A.); [email protected] (L.D.); [email protected] (P.D.); [email protected] (J.T.) 2 Faculty of Pharmacy, Université de Montréal, Montreal, QC H3C 3J7, Canada * Correspondence: [email protected]; Tel.: +1-856-938-8697 Received: 5 August 2020; Accepted: 31 August 2020; Published: 4 September 2020 Abstract: In an ageing society, polypharmacy has become a major public health and economic issue. Overuse of medications, especially in patients with chronic diseases, carries major health risks. One common consequence of polypharmacy is the increased emergence of adverse drug events, mainly from drug–drug interactions. The majority of currently available drugs are metabolized by CYP450 enzymes. Interactions due to shared CYP450-mediated metabolic pathways for two or more drugs are frequent, especially through reversible or irreversible CYP450 inhibition. The magnitude of these interactions depends on several factors, including varying affinity and concentration of substrates, time delay between the administration of the drugs, and mechanisms of CYP450 inhibition. Various types of CYP450 inhibition (competitive, non-competitive, mechanism-based) have been observed clinically, and interactions of these types require a distinct clinical management strategy. This review focuses on mechanism-based inhibition, which occurs when a substrate forms a reactive intermediate, creating a stable enzyme–intermediate complex that irreversibly reduces enzyme activity. -

Study Protocol
Full title: A Sequential Phase I study of MEK1/2 inhibitors PD-0325901 or Binimetinib combined with cMET inhibitor PF-02341066 in Patients with RAS Mutant and RAS Wild Type (with aberrant c-MET) Colorectal Cancer Short title: MErCuRIC1: MEK and MET Inhibition in Colorectal Cancer Protocol Version & date: Version 9.0; 29Oct2018 Sponsor Protocol Number: OCTO_049 Ethics Number: 14/SC/1010 EudraCT Number: 2014-000463-40 Funding Reference Number 602901-2 Sponsored by the University of Oxford Funder: European Commission’s Seventh Framework Program (FP7) Confidentiality Statement This document contains confidential information that must not be disclosed to anyone other than the Sponsor, the Trials Office, the Investigator Team, host NHS Trust(s), regulatory authorities, and members of the Research Ethics Committee. MErCuRIC1_Protocol_V9.0_29Oct2018 Protocol Template V3.0_18Feb2013 Page 1 of 121 MErCuRIC1 Confidential GENERAL CONTACT INFORMATION Trial Office (OCTO) MErCuRIC Trial Office Oncology Clinical Trials Office (OCTO) Department of Oncology, The University of Oxford Oxford Cancer and Haematology Centre Churchill Hospital Oxford Tel: +44 (0)1865 227194 Email: [email protected] Website: http://www.oncology.ox.ac.uk/research/oncology- clinical-trials-office-octo Chief Investigator Professor Mark Middleton Department of Oncology, University of Oxford Oxford Cancer and Haematology Centre Churchill Hospital Oxford OX3 7LE Tel: +44 (0)1865 235 315 Email : [email protected] Investigator Professor Richard Wilson and -

Effects of Synergistic Inhibition on Α-Glucosidase by Phytoalexins In
biomolecules Article Effects of Synergistic Inhibition on α-glucosidase by Phytoalexins in Soybeans Hyeong-U Son 1 , Eun-Kyeong Yoon 1, Chi-Yeol Yoo 1, Chul-Hong Park 2, Myung-Ae Bae 3, Tae-Ho Kim 4, Chang Hyung Lee 5, Ki Won Lee 5, Hogyun Seo 6, Kyung-Jin Kim 6 and Sang-Han Lee 1,7,* 1 School of Food Science & Biotechnology, Graduate School, Kyungpook National University, Daegu 41566, Korea; [email protected] (H.-U.S.); [email protected] (E.-K.Y.); [email protected] (C.-Y.Y.) 2 Pennington Biomedical Research Center, Louisiana State University, Baton Rouge, LA 70808, USA; [email protected] 3 Korea Research Institute of Chemical Technology, Daejeon 34114, Korea; [email protected] 4 Biomedical Research Institute, Kyungpook National University Hospital, Daegu 41940, Korea; [email protected] 5 Major in Biomodulation, Department of Agricultural Biotechnology and Research Institute for Agriculture and Life Sciences, Seoul National University, Seoul 08826, Korea; [email protected] (C.H.L.); [email protected] (K.W.L.) 6 School of Life Sciences, KNU Creative BioResearch Group, Institute of Microorganisms, Kyungpook National University, Daegu 41566, Korea; [email protected] (H.S.); [email protected] (K.-J.K.) 7 knu BnC, Daegu 41566, Korea * Correspondence: [email protected] Received: 18 October 2019; Accepted: 3 December 2019; Published: 5 December 2019 Abstract: To determine the mechanism of action of the effects of phytoalexins in soybeans, we analyzed α-glucosidase inhibition kinetics using Michaelis–Menten plots and Lineweaver–Burk plots. The results showed that the type of inhibition with glyceollin was competitive, that of genistein was noncompetitive, that of daidzein was uncompetitive, and luteolin showed a mixed mode of action. -

Mechanism of Enzymes Involved in Leukotriene C4 Biosynthesis
From Department of Medical Biochemistry and Biophysics Karolinska Institutet, Stockholm, Sweden MECHANISM OF ENZYMES INVOLVED IN LEUKOTRIENE C4 BIOSYNTHESIS H. R. Shabbir Ahmad Stockholm 2016 All previously published papers were reproduced with permission from the publisher. Published by Karolinska Institutet. Printed by E-Print AB © H. R. Shabbir Ahmad, 2016 ISBN 978-91-7676-368-1 Mechanism of Enzymes Involved in Leukotriene C4 Biosynthesis THESIS FOR DOCTORAL DEGREE (Ph.D.) By H. R. Shabbir Ahmad Opponent: Principal Supervisor: Tim Mantle, PhD Professor Jesper Z. Haeggström Trinity College Dublin Karolinska Institutet Ireland. Department of Medical Biochemistry and Biophysics Division of Chemistry II Examination Board: Co-supervisor(s): Professor Hans-Erik Claesson Karolinska Institutet Agnes Rinaldo-Matthis, PhD Department of Medicine, Solna. Karolinska Institutet Department of Medical Biochemistry and Professor Pia Ädelroth Biophysics Stockholm University Division of Chemistry II Department of Biochemistry and Biophysics Professor Ralf Morgenstern Docent Ylva Ivarsson Karolinska Institutet Uppsala University Institute of Environmental Medicine Department of Chemistry, BMC To my family ABSTRACT Cysteinyl leukotrienes (cys-LTs) are potent proinflammatory mediators associated with various diseases including asthma and allergic rhinitis. Leukotriene C4 synthase (LTC4S) and microsomal glutathione transferase 2 (MGST2) catalyze conjugation of the epoxide intermediate LTA4 with GSH to form LTC4, the parent compound of the cys-LTs. Both enzymes belong to the Membrane-Associated Proteins in Eicosanoid and Glutathione metabolism (MAPEG) super family of integral membrane proteins involved in the generation of lipid mediators and in the metabolism of xenobiotics. This thesis investigates the catalytic mechanism and regulation of LTC4S and MGST2. MGST2 can also catalyze conjugation of glutathione (GSH) with electrophilic substrates, such as 1-chloro-2,4-dinitrobenzene (CDNB) and also possesses GSH-dependent peroxidase activity. -

Enzymes: the Biological Accelerators
Organic and Medicinal Chemistry International Journal ISSN 2474-7610 Review Article Organic & Medicinal Chem IJ Volume 2 Issue 4 - May 2017 Copyright © All rights are reserved by Ravindra K. Rawal DOI: 10.19080/OMCIJ.2016.01.555594 Enzymes: The Biological Accelerators Sundeep Kaur Manjal, Ramandeep Kaur, Rohit Bhatia and Ravindra K. Rawal* Department of Pharmaceutical Chemistry, ISF College of Pharmacy, India Submission: April 06, 2017; Published: May 26,2017 *Corresponding author: Ravindra K. Rawal, Department of Pharmaceutical Chemistry, ISF College of Pharmacy, GT Road Moga, Punjab 142001, India, Tel: ; Email: Abstract Enzymes are the macromolecular biological catalysts which tend to exhibit tremendous biological value for the human society. These are known to accelerate and catalyze the chemical reactions many times faster than ordinary. Generally, they are known to catalyze more than 5,000 types of biochemical reactions. Numerous enzymes are produced inside the human body which tend to play crucial role in the functioning of biological activities. Some of the enzymes are used commercially such as for the synthesis of antibiotics and also used for household purpose like in manufacturing of washing powder. Apart from this, enzymes serve a variety of functions inside the living organisms. Recently pro-drug approach has gained wide popularity, it is mainly utilised in the lead optimization of the drug molecule. In this review we have tried to discuss different aspects of enzymes, enzyme kinetics, enzyme inhibitors, pro-drugs and their utility with suitable examples. Keywords: Enzyme; Serine; Trypsinogen; Heme; Pro-drug Introduction function are determined by four structural features that are - the Enzymes act as catalysts for almost all of the chemical metal core, the metal binding motif, the second sphere residues reactions that occur in all living organisms [1]. -

IF102-1B – RGGT Inhibition
View metadata, citation and similar papers at core.ac.uk brought to you by CORE provided by Access to Research and Communications Annals Phosphonocarboxylates inhibit the second geranylgeranyl addition by Rab Geranylgeranyl Transferase Rudi A. Baron1*, Richard Tavaré1§, Ana C. Figueiredo1, Katarzyna Błażewska2, Boris A. Kashemirov2, Charles E. McKenna2, Frank H. Ebetino3, Adam Taylor4, Michael J. Rogers4, Fraser P. Coxon4, and Miguel C. Seabra1,5,6¶ 1 Molecular Medicine, National Heart and Lung Institute, Imperial College London, London SW7 2AZ, UK, 2 Department of Chemistry, University of Southern California, Los Angeles, California 90089-0744, 3 Procter & Gamble Pharmaceuticals, Cincinnati, Ohio, USA, 4 Bone & Musculoskeletal Programme, Institute of Medical Sciences, University of Aberdeen, Aberdeen, AB25 2ZD, UK, 5Instituto Gulbenkian de Ciência, 2780-156 Oeiras, Portugal, 6Faculdade de Ciências Médicas, Universidade Nova de Lisboa, 1169-056 Lisboa, Portugal * Present address: Cerenis Therapeutics, 31682 Labège, France § Present address: Kings College London, Division of Imaging sciences, The Rayne Institute, 4th floor, Lambeth Wing, St. Thomas Hospital, London SE1 7EH ¶Address correspondence to Miguel C. Seabra: email: [email protected] Running title: Mechanism of RGGT inhibition by bisphosphonates Rab geranylgeranyl transferase demonstrate directly that in the presence of (RGGT) catalyzes the post-translational (+)-3-IPEHPC, Rab-CC and Rab-CXC geranylgeranyl (GG) modification of (usually) proteins are modified by only a single GG two C-terminal cysteines in Rab GTPases. addition. The presence of (+)-3-IPEHPC Here we studied the mechanism of the Rab resulted in a preference for the Rab N- geranylgeranylation reaction by terminal cysteine to be modified first, bisphosphonate analogs in which one suggesting an order of cysteine phosphonate group is replaced by a geranylgeranylation in RGGT catalysis. -

Recent Discoveries in the Genetics of Melanoma and Their Therapeutic Implications
Recent discoveries in the genetics of melanoma and their therapeutic implications. Amélie Marquette, Martine Bagot, Armand Bensussan, Nicolas Dumaz To cite this version: Amélie Marquette, Martine Bagot, Armand Bensussan, Nicolas Dumaz. Recent discoveries in the ge- netics of melanoma and their therapeutic implications.. Archivum Immunologiae et Therapiae Exper- imentalis, Springer Verlag, 2007, 55 (6), pp.363-72. 10.1007/s00005-007-0043-5. inserm-00195416 HAL Id: inserm-00195416 https://www.hal.inserm.fr/inserm-00195416 Submitted on 10 Dec 2007 HAL is a multi-disciplinary open access L’archive ouverte pluridisciplinaire HAL, est archive for the deposit and dissemination of sci- destinée au dépôt et à la diffusion de documents entific research documents, whether they are pub- scientifiques de niveau recherche, publiés ou non, lished or not. The documents may come from émanant des établissements d’enseignement et de teaching and research institutions in France or recherche français ou étrangers, des laboratoires abroad, or from public or private research centers. publics ou privés. Recent discoveries in the genetics of melanoma and their therapeutic implications Marquette Amélie 1 , Bagot Martine 1 2 , Bensussan Armand 1 , Dumaz Nicolas 1 * 1 IMRB, Institut Mondor de recherche biomédicale INSERM : U841, Université Paris XII Val de Marne, Hôpital Henri Mondor 51, av du mal de lattre de tassigny 94010 CRETEIL CEDEX,FR 2 Service de dermatologie Hôpital Henri Mondor, AP-HP, FR * Correspondence should be adressed to: Nicolas Dumaz <[email protected]> Abstract The incidence of cutaneous malignant melanoma, tumours arising from melanocytes, has increased markedly over the past few years in many countries. -

Studies of Reversible Inhibition, Irreversible Inhibition and Activation of Alkaline Phosphatase by Capillary Electrophoresis
University of Tennessee, Knoxville TRACE: Tennessee Research and Creative Exchange Masters Theses Graduate School 5-2004 Studies of Reversible Inhibition, Irreversible Inhibition and Activation of Alkaline Phosphatase by Capillary Electrophoresis Angela Randall Whisnant University of Tennessee, Knoxville Follow this and additional works at: https://trace.tennessee.edu/utk_gradthes Part of the Chemistry Commons Recommended Citation Whisnant, Angela Randall, "Studies of Reversible Inhibition, Irreversible Inhibition and Activation of Alkaline Phosphatase by Capillary Electrophoresis. " Master's Thesis, University of Tennessee, 2004. https://trace.tennessee.edu/utk_gradthes/4828 This Thesis is brought to you for free and open access by the Graduate School at TRACE: Tennessee Research and Creative Exchange. It has been accepted for inclusion in Masters Theses by an authorized administrator of TRACE: Tennessee Research and Creative Exchange. For more information, please contact [email protected]. To the Graduate Council: I am submitting herewith a thesis written by Angela Randall Whisnant entitled "Studies of Reversible Inhibition, Irreversible Inhibition and Activation of Alkaline Phosphatase by Capillary Electrophoresis." I have examined the final electronic copy of this thesis for form and content and recommend that it be accepted in partial fulfillment of the equirr ements for the degree of Master of Science, with a major in Chemistry. S. Douglass Gilman, Major Professor We have read this thesis and recommend its acceptance: James Q. Chambers, David C. Baker Accepted for the Council: Carolyn R. Hodges Vice Provost and Dean of the Graduate School (Original signatures are on file with official studentecor r ds.) To the Graduate Council: I am submitting herewith a thesis written by Angela Whisnant entitled "Studies of Reversible Inhibition, Irreversible Inhibition and Activation of Alkaline Phosphatase by Capillary Electrophoresis". -

Chapter 8. Enzymes: Basic Concepts and Kinetics
CHAPTER 8. ENZYMES: BASIC CONCEPTS AND KINETICS coelenterazine The activity of an enzyme is responsible for the glow of the luminescent jellyfish at left. The enzyme aequorin catalyzes the oxidation of a compound by oxygen in the presence of calcium to release CO2 and light CHAPTER 8. ENZYMES: BASIC CONCEPTS AND KINETICS Introduction . Enzymes are large biological molecules that catalyze chemical transformations . The molecule also mediates the transformation of one form of energy into another . 25% of the genes in the human genome encode enzymes . Catalytic power and specificity – important characteristics . Active site – the place where enzymatic reactions take place CHAPTER 8. ENZYMES: BASIC CONCEPTS AND KINETICS Introduction . Nearly all known enzymes are proteins • RNA can function as a catalyst . Proximity effect – enzymes bring substrates closer together in an optimal orientation . They catalyze reactions by stabilizing transition states 8.1 CATALYTIC POWER AND SPECIFICITY Rate enhancement by enzymes . Enzymes accelerate reactions by factors of as much as a million or more 8.1 CATALYTIC POWER AND SPECIFICITY Substrate specificity . A substrate is a molecule upon which an enzyme acts . Some proteases cleaves specific amide bonds in a peptide . The specificity of an enzyme is due to the precise interaction of the substrate with the enzyme . This precision is a result of the intricate 3D structure of the enzyme protein Fig 8.1 Enzyme specificity. (A) Trypsin cleaves on the carboxyl side of arginine and lysine residues, whereas (B) thrombin cleaves Arg-Gly bonds in particular sequences only 8.1 CATALYTIC POWER AND SPECIFICITY Cofactors . The catalytic activity of enzymes depends on the presence of small molecules termed cofactors . -

Phase I Enzyme Inhibition
5 Phase I Enzyme Inhibition 5.1 Introduction The previous chapter was mostly aimed at problems associated with drug failure due to enzyme induction. However, when drug clearance is slowed or even stopped for any reason, the consequences are more dangerous and occur much more rapidly compared with enzyme induction. Generally, the phar- macological effects of the drugs will be greatly intensified, leading to a clear manifestation of symptoms in the patient. In drugs with a high therapeutic index, this may not be a problem and the effects of the drug accumulation will be reversible. In narrow therapeutic index drugs, the effects can be lethal in hours. In other cases, a drug may induce a potentially lethal pharmaco- logical effect that is only seen in very high doses, way above the normal range. This effect may or may not have been seen in the initial pre-clinical (animal) toxicity testing of the drug. The following illustrative histories underline the effects of drug accumulation. History 1 A previously healthy 29-year-old male used terfenadine twice daily for one year to treat allergic rhinitis. The patient drank grapefruit juice two to three times weekly. On the day of his death, he consumed two glasses of juice, took his terfenadine dose, and then mowed his lawn; within one hour he became ill, collapsed and died. Although usually undetectable, post-mortem terfena- dine and terfenadine metabolite plasma levels were reported as 35 and 130 ng/mL respectively. These levels are within range of previously noted arrhyth- mogenic levels of terfenadine. The individual had no evidence of impaired hepatic function.